Nanomaterial-based catalyst
Nanomaterial-based catalysts are usually heterogeneous catalysts broken up into metal nanoparticles in order to enhance the catalytic process. Metal nanoparticles have high surface area, which can increase catalytic activity. Nanoparticle catalysts can be easily separated and recycled.[1][2][3] They are typically used under mild conditions to prevent decomposition of the nanoparticles.[4]
Functionalized nanoparticles
Functionalized metal nanoparticles are more stable toward solvents compared to non-functionalized metal nanoparticles.[5][6] In liquids, the metal nanoparticles can be affected by van der Waals force. Particle aggregation can sometimes decrease catalytic activity by lowering the surface area.[7] Nanoparticles can also be functionalized with polymers or oligomers to sterically stabilize the nanoparticles by providing a protective layer that prevents the nanoparticles from interacting with each other.[8] Alloys of two metals, called bimetallic nanoparticles, are used to create synergistic effects on catalysis between the two metals.[9]
Potential applications
Dehalogenation and hydrogenation
Nanoparticle catalysts are active for the hydrogenolysis of C-Cl bonds such as polychlorinated biphenyls.[5][6] Another reaction is hydrogenation of halogenated aromatic amines is also important for the synthesis of herbicides and pesticides as well as diesel fuel.[5] In organic chemistry, hydrogenation of a C-Cl bond with deuterium is used to selectively label the aromatic ring for use in experiments dealing with the kinetic isotope effect. Buil et al. created rhodium complexes that generated rhodium nanoparticles. These nanoparticles catalyzed the dehalogenation of aromatic compounds as well as the hydrogenation of benzene to cyclohexane.[6] Polymer-stabilized nanoparticles can also be used for the hydrogenation of cinnamaldehyde and citronellal.[5][7][10][9] Yu et al. found that the ruthenium nanocatalysts are more selective in the hydrogenation of citronellal compared to the traditional catalysts used.[9]
Hydrosilylation reactions
The Reduction of gold, cobalt, nickel, palladium, or platinum organometallic complexes with silanes produces metal nanoparticle that catalyze the hydrosilylation reaction.[11] BINAP-functionalized palladium nanoparticles and gold nanoparticles have been used for the hydrosilylaytion of styrene under mild conditions; they were found to be more catalytically active and more stable than non-nanoparticle Pd-BINAP complexes.[11][12] The reaction may also be catalyzed by a nanoparticle that consists of two metals.[5][13]
Organic redox reactions
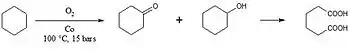
An oxidation reaction to form adipic acid is shown in figure 3 and it can be catalyzed by cobalt nanoparticles.[5] This is used in an industrial scale to produce the nylon 6,6 polymer. Other examples of oxidation reactions that are catalyzed by metallic nanoparticles include the oxidation of cyclooctane, the oxidation of ethene, and glucose oxidation.[5]
C-C coupling reactions
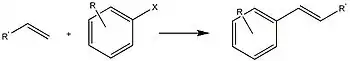
Metallic nanoparticles can catalyze C–C coupling reactions such as the hydroformylation of olefins,[5] the synthesis of vitamin E and the Heck coupling and Suzuki coupling reactions.[5]
Palladium nanoparticles were found to efficiently catalyze Heck coupling reactions. It was found that increased electronegativity of the ligands on the palladium nanoparticles increased their catalytic activity.[5][14]
The compound Pd2(dba)3 is a source of Pd(0), which is the catalytically active source of palladium used for many reactions, including cross coupling reactions.[4] Pd2(dba)3 was thought to be a homogeneous catalytic precursor, but recent articles suggest that palladium nanoparticles are formed, making it a heterogeneous catalytic precursor.[4]
Alternative fuels
Iron oxide and cobalt nanoparticles can be loaded onto various surface active materials like alumina to convert gases such as carbon monoxide and hydrogen into liquid hydrocarbon fuels using the Fischer-Tropsch process.[15][16]
Much research on nanomaterial-based catalysts has to do with maximizing the effectiveness of the catalyst coating in fuel cells. Platinum is currently the most common catalyst for this application, however, it is expensive and rare, so a lot of research has been going into maximizing the catalytic properties of other metals by shrinking them to nanoparticles in the hope that someday they will be an efficient and economic alternative to platinum. Gold nanoparticles also exhibit catalytic properties, despite the fact that bulk gold is unreactive.
Yttrium stabilized zirconium nanoparticles were found to increase the efficiency and reliability of a solid oxide fuel cell.[17][18] Nanomaterial ruthenium/platinum catalysts could potentially be used to catalyze the purification of hydrogen for hydrogen storage.[19] Palladium nanoparticles can be functionalized with organometallic ligands to catalyze the oxidation of CO and NO to control air pollution in the environment.[17] Carbon nanotube supported catalysts can be used as a cathode catalytic support for fuel cells and metal nanoparticles have been used to catalyze the growth of carbon nanotubes.[17] Platinum-cobalt bimetallic nanoparticles combined with carbon nanotubes are promising candidates for direct methanol fuel cells since they produce a higher stable current electrode.[17]
Medicine
In magnetic chemistry, nanoparticles can be used for catalyst support for medicinal use.
Nanozymes
Besides conventional catalysis, nanomaterials have been explored for mimicking natural enzymes. The nanomaterials with enzyme mimicking activities are termed as nanozymes.[20] Many nanomaterials have been used to mimic varieties of natural enzymes, such as oxidase, peroxidase, catalase, SOD, nuclease, etc. The nanozymes have found wide applications in many areas, from biosensing and bioimaging to therapeutics and water treatment.
Nanostructures for electrocatalysis
Nanocatalysts are of wide interest in fuel cells and electrolyzers, where the catalyst strongly affects efficiency.
Nanoporous surfaces
In fuel cells, nanoporous materials are widely used to make cathodes. Porous nanoparticles of platinum have good activity in nanocatalysis but are less stable and their lifetime is short.[21]
Nanoparticles
One drawback to the use of nanoparticles is their tendency to agglomerate. The problem can be mitigated with the correct catalyst support. Nanoparticles are optimal structures to be used as nanosensors because they can be tuned to detect specific molecules. Examples of Pd nanoparticles electrodeposited on multi-walled carbon nanotubes have shown good activity towards catalysis of cross-coupling reactions.[22]
Nanowires
Nanowires are very interesting for electrocatalytic purpose because they are easier to produce and the control over their characteristics in the production process is quite precise. Also, nanowires can increase faradaic efficiency due to their spatial extent and thus to greater availability of reactants on the active surface.[23]
Materials
The nanostructures involved in electrocatalysis processes can be made up of different materials. Through the use of nanostructured materials, electrocatalysts can achieve good physical-chemical stability, high activity, good conductivity and low cost. Metallic nanomaterials are commonly made up of transition metals (mostly iron, cobalt, nickel, palladium, platinum). Multi-metal nanomaterials show new properties due to the characteristics of each metal. The advantages are the increase in activity, selectivity and stability and the cost reduction. Metals can be combined in different ways such as in the core-shell bimetallic structure: the cheapest metal forms the core and the most active one (typically a noble metal) constitutes the shell. By adopting this design, the use of rare and expensive metals can be reduced down to 20%.[24]
One of the future challenges is to find new stable materials, with good activity and especially low cost. Metallic glasses, polymeric carbon nitride (PCN) and materials derived from metal-organic frameworks (MOF) are just a few examples of materials with electrocatalytic properties on which research is currently investing.[25][26][27]
Photocatalysis
Many of the photocatalytic systems can benefit from the coupling with a noble metal; the first Fujishima-Honda cell made use of a co-catalyst plate as well. For instance, the essential design of a disperse photocatalytic reactor for water splitting is that of a water sol in which the dispersed phase is made up of semiconductor quantum dots each coupled to a metallic co-catalyst: the QD converts the incoming electromagnetic radiation into an exciton whilst the co-catalyst acts as an electron scavenger and lowers the overpotential of the electrochemical reaction.[28]
Characterization of nanoparticles
Some techniques that can be used to characterize functionalized nanomaterial catalysts include X-ray photoelectron spectroscopy, transmission electron microscopy, circular dichroism spectroscopy, nuclear magnetic resonance spectroscopy, UV-visible spectroscopy and related experiments.
See also
References
- Bahrami, Foroogh; Panahi, Farhad; Daneshgar, Fatemeh; Yousefi, Reza; Shahsavani, Mohammad Bagher; Khalafi-nezhad, Ali (2016). "Synthesis of new α-aminophosphonate derivatives incorporating benzimidazole, theophylline and adenine nucleobases using l-cysteine functionalized magnetic nanoparticles (LCMNP) as magnetic reusable catalyst: evaluation of their anticancer properties". RSC Advances. 6 (7): 5915–5924. doi:10.1039/C5RA21419J.
- Fukui, Takehisa; Murata, Kenji; Ohara, Satoshi; Abe, Hiroya; Naito, Makio; Nogi, Kiyoshi (2004). "Morphology control of Ni–YSZ cermet anode for lower temperature operation of SOFCs". Journal of Power Sources. 125 (1): 17–21. Bibcode:2004JPS...125...17F. doi:10.1016/S0378-7753(03)00817-6.
- Pierluigi Barbaro, Francesca Liguori, ed. (2010). Heterogenized homogeneous catalysts for fine chemicals production : materials and processes. Dordrecht: Springer. ISBN 978-90-481-3695-7.
- Zalesskiy, Sergey; Ananikov Valentine (March 2012). "Pd2(dba)3 as a Precursor of Soluble Metal Complexes and Nanoparticles: Determination of Palladium Active Species for Catalysis and Synthesis". Organometallics. 31 (6): 2302–2309. doi:10.1021/om201217r.
- Panahi, Farhad; Bahrami, Foroogh; Khalafi-nezhad, Ali (2017). "Magnetic nanoparticles grafted l-carnosine dipeptide: remarkable catalytic activity in water at room temperature". Journal of the Iranian Chemical Society. 14 (10): 2211–2220. doi:10.1007/s13738-017-1157-2. S2CID 103858148.
- Roucoux, Alain; Schulz, Jürgen; Patin, Henri (2002). "Reduced Transition Metal Colloids: A Novel Family of Reusable Catalysts?". Chemical Reviews. 102 (10): 3757–3778. doi:10.1021/cr010350j. PMID 12371901.
- Yu, Weiyong; Liu, Hanfan; Liu, Manhong; Liu, Zhijie (2000). "Selective hydrogenation of citronellal to citronellol over polymer-stabilized noble metal colloids". Reactive and Functional Polymers. 44 (1): 21–29. doi:10.1016/S1381-5148(99)00073-5.
- Buil, María L.; Esteruelas, Miguel A.; Niembro, Sandra; Oliván, Montserrat; Orzechowski, Lars; Pelayo, Cristina; Vallribera, Adelina (2010). "Dehalogenation and Hydrogenation of Aromatic Compounds Catalyzed by Nanoparticles Generated from Rhodium Bis(imino)pyridine Complexes". Organometallics. 29 (19): 4375–4383. doi:10.1021/om1003072. hdl:10261/52564.
- Yu, W; Liu, M; Liu, H; Ma, X; Liu, Z (1998). "Preparation, Characterization, and Catalytic Properties of Polymer-Stabilized Ruthenium Colloids". Journal of Colloid and Interface Science. 208 (2): 439–444. Bibcode:1998JCIS..208..439Y. doi:10.1006/jcis.1998.5829. PMID 9845688.
- Yu, Weiyong; Liu, Manhong; Liu, Hanfan; An, Xiaohua; Liu, Zhijie; Ma, Xiaoming (1999). "Immobilization of polymer-stabilized metal colloids by a modified coordination capture: preparation of supported metal colloids with singular catalytic properties". Journal of Molecular Catalysis A: Chemical. 142 (2): 201–211. doi:10.1016/S1381-1169(98)00282-9.
- Tamura, Masaru; Fujihara, Hisashi (2003). "Chiral Bisphosphine BINAP-Stabilized Gold and Palladium Nanoparticles with Small Size and Their Palladium Nanoparticle-Catalyzed Asymmetric Reaction". Journal of the American Chemical Society. 125 (51): 15742–15743. doi:10.1021/ja0369055. PMID 14677954.
- Leeuwen, Piet W.N.M. van; Chadwick, John C. (5 July 2011). Homogeneous catalysts : activity, stability, deactivation. Weinheim, Germany: Wiley -VCH. ISBN 978-3-527-32329-6.
- Lewis, Larry N.; Lewis, Nathan. (1986). "Platinum-catalyzed hydrosilylation – colloid formation as the essential step". Journal of the American Chemical Society. 108 (23): 7228–7231. doi:10.1021/ja00283a016.
- Beller, Matthias; Fischer, Hartmut; Kühlein, Klaus; Reisinger, C.-P.; Herrmann, W.A. (1996). "First palladium-catalyzed Heck reactions with efficient colloidal catalyst systems". Journal of Organometallic Chemistry. 520 (1–2): 257–259. doi:10.1016/0022-328X(96)06398-X.
- Vengsarkar, Pranav S.; Xu, Rui; Roberts, Christopher B. (2015-12-02). "Deposition of Iron Oxide Nanoparticles onto an Oxidic Support Using a Novel Gas-Expanded Liquid Process to Produce Functional Fischer–Tropsch Synthesis Catalysts". Industrial & Engineering Chemistry Research. 54 (47): 11814–11824. doi:10.1021/acs.iecr.5b03123. ISSN 0888-5885.
- Khodakov, Andrei Y.; Chu, Wei; Fongarland, Pascal (2007-05-01). "Advances in the Development of Novel Cobalt Fischer−Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels". Chemical Reviews. 107 (5): 1692–1744. doi:10.1021/cr050972v. ISSN 0009-2665. PMID 17488058.
- Moshfegh, A Z (2009). "Nanoparticle catalysts". Journal of Physics D: Applied Physics. 42 (23): 233001. Bibcode:2009JPhD...42w3001M. doi:10.1088/0022-3727/42/23/233001.
- Ananikov, Valentine P.; Orlov, Nikolay V.; Beletskaya, Irina P. (2007). "Highly Efficient Nickel-Based Heterogeneous Catalytic System with Nanosized Structural Organization for Selective Se−H Bond Addition to Terminal and Internal Alkynes". Organometallics. 26 (3): 740–750. doi:10.1021/om061033b.
- Beal, James. "New nanoparticle catalyst brings fuel-cell cars closer to showroom". University of Wisconsin at Madison. Retrieved 20 March 2012.
- Wei, Hui; Wang, Erkang (2013-06-21). "Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes". Chemical Society Reviews. 42 (14): 6060–93. doi:10.1039/C3CS35486E. ISSN 1460-4744. PMID 23740388. S2CID 39693417.
- Bae, J.H.; Han, J.H.; Chung, T.D. (2012). "Electrochemistry at nanoporous interfaces: new opportunity for electrocatalysis". Physical Chemistry Chemical Physics. 14 (2): 448–463. Bibcode:2012PCCP...14..448B. doi:10.1039/C1CP22927C. PMID 22124339.
- Radtke, Mariusz (1 July 2015). "Electrodeposited palladium on MWCNTs as "semi-soluble heterogeneous" catalyst for cross-coupling reactions". Tetrahedron Letters. 56 (27): 4084. doi:10.1016/j.tetlet.2015.05.019.
- Mistry, H.; Varela, A.S.; Strasser, P.; Cuenya, B.R. (2016). "Nanostructured electrocatalysts with tunable activity and selectivity". Nature Reviews Materials. 1 (4): 1–14. Bibcode:2016NatRM...116009M. doi:10.1038/natrevmats.2016.9.
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; Toney, M.F.; Nilsson, A. (2010). "Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts". Nature Chemistry. 2 (6): 454–460. Bibcode:2010NatCh...2..454S. doi:10.1038/nchem.623. PMID 20489713.
- Hu, Y.C.; Sun, C.; Sun, C. (2019). "Functional Applications of Metallic Glasses in Electrocatalysis". ChemCatChem. 11 (10): 2401–2414. doi:10.1002/cctc.201900293. S2CID 132328392.
- Wang, Z.; Hu, X.; Zou, G.; Huang, Z.; Tang, Z.; Liu, Q.; Hu, G.; Geng, D. (2019). "Advances in constructing polymeric carbon-nitride-based nanocomposites and their applications in energy chemistry". Sustainable Energy & Fuels. 3 (3): 611–655. doi:10.1039/C8SE00629F.
- Liu, X.; Wu, Y.; Guan, C.; Cheetham, A.K.; Wang, J. (2018). "MOF-derived nanohybrids for electrocatalysis and energy storage: current status and perspectives". Chemical Communications. 54 (42): 5268–5288. doi:10.1039/C8CC00789F. PMID 29582028.
- Chen, S.; Takata, T.; Domen, K. (2017). "Particulate photocatalysts for overall water splitting". Nature Reviews Materials. 2 (10): 17050. Bibcode:2017NatRM...217050C. doi:10.1038/natrevmats.2017.50.
Further reading
- Santen, Rutger Anthony van; Neurock, Matthew (2006). Molecular heterogeneous catalysis : a conceptual and computational approach ([Online-Ausg.] ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-29662-0.