N-Oleyl-1,3-propanediamine
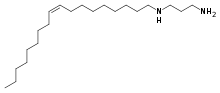
N-Oleyl-1,3-propanediamine is an organic compound and a diamine with the formula C21H44N2.[2][3] It has found use in numerous industries. The main producer of commercial N-Oleyl-1,3-propanediamine is AkzoNobel, who sells it under the name Duomeen OL.[4]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N1-[(9Z)-Octadec-9-en-1-yl]propane-1,3-diamine | |
| Other names
(Z)-N-9-octadecenylpropane-1,3-diamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.027.754 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H44N2 | |
| Molar mass | 324.597 g·mol−1 |
| Appearance | Colourless or yellow liquid |
| Odor | Ammoniacal |
| Density | 0.841 g/cm3 |
| Melting point | 12 °C (54 °F; 285 K) |
| Boiling point | 300 °C (572 °F; 573 K) |
| insoluble (at 20 °C) | |
| Solubility | Soluble in acetone, methanol |
| Hazards | |
| GHS labelling: | |
     | |
| Danger | |
| H290, H301, H302, H315, H318, H372, H410 | |
| P234, P260, P264, P270, P273, P280, P301+P330+P331, P302+P352, P304+P340, P321, P330, P362+P364, P363, P390, P391, P405, P406, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 54 °C (129 °F; 327 K) |
| Safety data sheet (SDS) | [1] |
| Related compounds | |
Related amines |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uses
N-Oleyl-1,3-propanediamine is used as a catalyst in the production of urethanes and epoxies. It is used as a emulsifier in the making of asphalt, an ore flotation agent, and a dispersant for some paints. It has also found use as a lubricant due to its unreactivity with cations, which are present in some adhesive manufacturing.
References
- http://chem-international.com/wp-content/uploads/2013/08/Oleyl-Diamine.pdf
- "(Z)-N-9-octadecenylpropane-1,3-diamine | 7173-62-8". Chemicalbook.com. Retrieved 2022-05-02.
- National Center for Biotechnology Information. PubChem Compound Database; CID=40764. https://pubchem.ncbi.nlm.nih.gov/compound/40764 (accessed 2016-12-24).
- "Duomeen OL - AkzoNobel Functional Applications". Archived from the original on 2017-11-27. Retrieved 2016-12-24.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
