Molozonide
A molozonide (or "molecular ozonide") is a 1,2,3-trioxolane, which can also be considered a cyclic disubstituted trioxidane derivative.[1] Molozonides are formed by cycloaddition of ozone and an alkene during ozonolysis, as a transient intermediate which quickly rearranges to give the ozonide (1,2,4-trioxolane), the relatively stable product generated immediately prior to reductive or oxidative cleavage to form alcohols, carbonyl compounds, or derivatives thereof.[2]
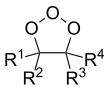
General chemical structure of molozonides
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "molozonides". doi:10.1351/goldbook.M04004
- McMurry, John (2004). Organic Chemistry, 6th ed. Belmont: Brooks/Cole. p. 225. ISBN 978-0-534-38999-4.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.