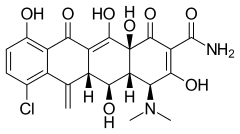
Meclocycline
Meclocycline (INN) is a tetracycline antibiotic.[1] It is used topically (i.e. for skin infections) as it is totally insoluble in water and may cause liver and kidney damage if given systemically.
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.309 |
| Chemical and physical data | |
| Formula | C22H21ClN2O8 |
| Molar mass | 476.87 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Its production for medical use has been discontinued.[2] It was previously sold in the United States by Pfizer under the brand name Meclan.[3]
References
- "Meclocycline sulfosalicylate". pubchem.ncbi.nlm.nih.gov. Retrieved 3 January 2019.
- "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". www.accessdata.fda.gov. Retrieved 3 January 2019.
- "MECLAN Drugs@FDA: FDA-Approved Drugs". www.accessdata.fda.gov. United States Food and Drug Administration. 11 August 2022. Retrieved 2022-08-11.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.