List of viscosities
Dynamic viscosity is a material property which describes the resistance of a fluid to shearing flows. It corresponds roughly to the intuitive notion of a fluid's 'thickness'. For instance, honey has a much higher viscosity than water. Viscosity is measured using a viscometer. Measured values span several orders of magnitude. Of all fluids, gases have the lowest viscosities, and thick liquids have the highest.
The values listed in this article are representative estimates only, as they do not account for measurement uncertainties, variability in material definitions, or non-Newtonian behavior.
Kinematic viscosity is dynamic viscosity divided by fluid density. This page lists only dynamic viscosity.
Units and conversion factors
For dynamic viscosity, the SI unit is Pascal-second. In engineering, the unit is usually Poise or centiPoise, with 1 Poise = 0.1 Pascal-second, and 1 centiPoise = 0.01 Poise.
For kinematic viscosity, the SI unit is m^2/s. In engineering, the unit is usually Stoke or centiStoke, with 1 Stoke = 0.0001 m^2/s, and 1 centiStoke = 0.01 Stoke.
For liquid, the dynamic viscosity is usually in the range of 0.001 to 1 Pascal-second, or 1 to 1000 centiPoise. The density is usually on the order of 1000 kg/m^3, i.e. that of water. Consequently, if a liquid has dynamic viscosity of n centiPoise, and its density is not too different from that of water, then its kinematic viscosity is around n centiStokes.
For gas, the dynamic viscosity is usually in the range of 10 to 20 microPascal-seconds, or 0.01 to 0.02 centiPoise. The density is usually on the order of 0.5 to 5 kg/m^3. Consequently, its kinematic viscosity is around 2 to 40 centiStokes.
Viscosities at or near standard conditions
Here "standard conditions" refers to temperatures of 25 °C and pressures of 1 atmosphere. Where data points are unavailable for 25 °C or 1 atmosphere, values are given at a nearby temperature/pressure.
The temperatures corresponding to each data point are stated explicitly. By contrast, pressure is omitted since gaseous viscosity depends only weakly on it.
Noble gases
The simple structure of noble gas molecules makes them amenable to accurate theoretical treatment. For this reason, measured viscosities of the noble gases serve as important tests of the kinetic-molecular theory of transport processes in gases (see Chapman–Enskog theory). One of the key predictions of the theory is the following relationship between viscosity , thermal conductivity , and specific heat :
where is a constant which in general depends on the details of intermolecular interactions, but for spherically symmetric molecules is very close to .[1]
This prediction is reasonably well-verified by experiments, as the following table shows. Indeed, the relation provides a viable means for obtaining thermal conductivities of gases since these are more difficult to measure directly than viscosity.[1][2]
| Substance | Molecular formula |
Viscosity (μPa·s) |
Thermal conductivity (W m−1K−1) |
Specific heat (J K−1kg−1) |
Notes | Refs. | |
|---|---|---|---|---|---|---|---|
| Helium | He | 19.85 | 0.153 | 3116 | 2.47 | [2][3] | |
| Neon | Ne | 31.75 | 0.0492 | 618 | 2.51 | [2][3] | |
| Argon | Ar | 22.61 | 0.0178 | 313 | 2.52 | [2][3] | |
| Krypton | Kr | 25.38 | 0.0094 | 149 | 2.49 | [2][3] | |
| Xenon | Xe | 23.08 | 0.0056 | 95.0 | 2.55 | [2][3] | |
| Radon | Rn | ≈26 | ≈0.00364 | 56.2 | T = 26.85 °C; calculated theoretically; estimated assuming |
[4] | |
Diatomic elements
| Substance | Molecular formula | Viscosity (μPa·s) | Notes | Ref. |
|---|---|---|---|---|
| Hydrogen | H2 | 8.90 | [5] | |
| Nitrogen | N2 | 17.76 | [5] | |
| Oxygen | O2 | 20.64 | [6] | |
| Fluorine | F2 | 23.16 | [7] | |
| Chlorine | Cl2 | 13.40 | [7] | |
Hydrocarbons
| Substance | Molecular formula | Viscosity (μPa·s) | Notes | Ref. |
|---|---|---|---|---|
| Methane | CH4 | 11.13 | [8] | |
| Acetylene | C2H2 | 10.2 | T = 20 °C | [9] |
| Ethylene | C2H4 | 10.28 | [8] | |
| Ethane | C2H6 | 9.27 | [8] | |
| Propyne | C3H4 | 8.67 | T = 20 °C | [9] |
| Propene | C3H6 | 8.39 | [10] | |
| Propane | C3H8 | 8.18 | [8] | |
| Butane | C4H10 | 7.49 | [8] |
Organohalides
| Substance | Molecular formula | Viscosity (μPa·s) | Notes | Ref. |
|---|---|---|---|---|
| Carbon tetrafluoride | CF4 | 17.32 | [11] | |
| Fluoromethane | CH3F | 11.79 | [12] | |
| Difluoromethane | CH2F2 | 12.36 | [12] | |
| Fluoroform | CHF3 | 14.62 | [12] | |
| Pentafluoroethane | C2HF5 | 12.94 | [12] | |
| Hexafluoroethane | C2F6 | 14.00 | [12] | |
| Octafluoropropane | C3F8 | 12.44 | [12] |
Other gases
| Substance | Molecular formula | Viscosity (μPa·s) | Notes | Ref. |
|---|---|---|---|---|
| Air | 18.46 | [6] | ||
| Ammonia | NH3 | 10.07 | [13] | |
| Nitrogen trifluoride | NF3 | 17.11 | T = 26.85 °C | [14] |
| Boron trichloride | BCl3 | 12.3 | Theoretical estimate at T = 26.85 °C; estimated uncertainty of 10% |
[14] |
| Carbon dioxide | CO2 | 14.90 | [15] | |
| Carbon monoxide | CO | 17.79 | [16] | |
| Hydrogen sulfide | H2S | 12.34 | [17] | |
| Nitric oxide | NO | 18.90 | [7] | |
| Nitrous oxide | N2O | 14.90 | [18] | |
| Sulfur dioxide | SO2 | 12.82 | [10] | |
| Sulfur hexafluoride | SF6 | 15.23 | [5] | |
| Molybdenum hexafluoride | MoF6 | 14.5 | Theoretical estimates at T = 26.85 °C | [19] |
| Tungsten hexafluoride | WF6 | 17.1 | ||
| Uranium hexafluoride | UF6 | 17.4 |
n-Alkanes
Substances composed of longer molecules tend to have larger viscosities due to the increased contact of molecules across layers of flow.[20] This effect can be observed for the n-alkanes and 1-chloroalkanes tabulated below. More dramatically, a long-chain hydrocarbon like squalene (C30H62) has a viscosity an order of magnitude larger than the shorter n-alkanes (roughly 31 mPa·s at 25 °C). This is also the reason oils tend to be highly viscous, since they are usually composed of long-chain hydrocarbons.
| Substance | Molecular formula | Viscosity (mPa·s) | Notes | Ref. |
|---|---|---|---|---|
| Pentane | C5H12 | 0.224 | [21] | |
| Hexane | C6H14 | 0.295 | [22] | |
| Heptane | C7H16 | 0.389 | [22] | |
| Octane | C8H18 | 0.509 | [22] | |
| Nonane | C9H20 | 0.665 | [21] | |
| Decane | C10H22 | 0.850 | [22] | |
| Undecane | C11H24 | 1.098 | [21] | |
| Dodecane | C12H26 | 1.359 | [22] | |
| Tridecane | C13H28 | 1.724 | [21] | |
| Tetradecane | C14H30 | 2.078 | [22] | |
| Pentadecane | C15H32 | 2.82 | T = 20 °C | [23] |
| Hexadecane | C16H34 | 3.03 | [21] | |
| Heptadecane | C17H36 | 4.21 | T = 20 °C | [24] |
1-Chloroalkanes
| Substance | Molecular formula | Viscosity (mPa·s) | Notes | Ref. |
|---|---|---|---|---|
| Chlorobutane | C4H9Cl | 0.4261 | [25] | |
| Chlorohexane | C6H11Cl | 0.6945 | ||
| Chlorooctane | C8H17Cl | 1.128 | ||
| Chlorodecane | C10H21Cl | 1.772 | ||
| Chlorododecane | C12H25Cl | 2.668 | ||
| Chlorotetradecane | C14H29Cl | 3.875 | ||
| Chlorohexadecane | C16H33Cl | 5.421 | ||
| Chlorooctadecane | C18H37Cl | 7.385 | Supercooled liquid |
Other halocarbons
| Substance | Molecular formula | Viscosity (mPa·s) | Notes | Ref. |
|---|---|---|---|---|
| Dichloromethane | CH2Cl2 | 0.401 | [26] | |
| Trichloromethane (chloroform) |
CHCl3 | 0.52 | [10] | |
| Tribromomethane (bromoform) |
CHBr3 | 1.89 | [27] | |
| Carbon tetrachloride | CCl4 | 0.86 | [27] | |
| Trichloroethylene | C2HCl3 | 0.532 | [28] | |
| Tetrachloroethylene | C2Cl4 | 0.798 | T = 30 °C | [28] |
| Chlorobenzene | C6H5Cl | 0.773 | [29] | |
| Bromobenzene | C6H5Br | 1.080 | [29] | |
| 1-Bromodecane | C10H21Br | 3.373 | [30] |
Alkenes
| Substance | Molecular formula | Viscosity (mPa·s) | Notes | Ref. |
|---|---|---|---|---|
| 2-Pentene | C5H10 | 0.201 | [31] | |
| 1-Hexene | C6H12 | 0.271 | [32] | |
| 1-Heptene | C7H14 | 0.362 | [32] | |
| 1-Octene | C8H16 | 0.506 | T = 20 °C | [31] |
| 2-Octene | C8H16 | 0.506 | T = 20 °C | [31] |
| n-Decene | C10H20 | 0.828 | T = 20 °C | [31] |
Other liquids
| Substance | Molecular formula | Viscosity (mPa·s) | Notes | Ref. |
|---|---|---|---|---|
| Acetic acid | C2H4O2 | 1.056 | [21] | |
| Acetone | C3H6O | 0.302 | [33] | |
| Benzene | C6H6 | 0.604 | [21] | |
| Bromine | Br2 | 0.944 | [21] | |
| Ethanol | C2H6O | 1.074 | [21] | |
| Glycerol | C3H8O3 | 1412 | [34] | |
| Hydrazine | H4N2 | 0.876 | [21] | |
| Iodine pentafluoride | IF5 | 2.111 | [35] | |
| Mercury | Hg | 1.526 | [21] | |
| Methanol | CH4O | 0.553 | [36] | |
| 1-Propanol (propyl alcohol) | C3H8O | 1.945 | [37] | |
| 2-Propanol (isopropyl alcohol) | C3H8O | 2.052 | [37] | |
| Squalane | C30H62 | 31.123 | [38] | |
| Water | H2O | 1.0016 | T = 20 °C, standard pressure | [21] |
Aqueous solutions
The viscosity of an aqueous solution can either increase or decrease with concentration depending on the solute and the range of concentration. For instance, the table below shows that viscosity increases monotonically with concentration for sodium chloride and calcium chloride, but decreases for potassium iodide and cesium chloride (the latter up to 30% mass percentage, after which viscosity increases).
The increase in viscosity for sucrose solutions is particularly dramatic, and explains in part the common experience of sugar water being "sticky".
| Table: Viscosities (in mPa·s) of aqueous solutions at T = 20 °C for various solutes and mass percentages[21] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solute | mass percentage = 1% | 2% | 3% | 4% | 5% | 10% | 15% | 20% | 30% | 40% | 50% | 60% | 70% |
| Sodium chloride (NaCl) | 1.020 | 1.036 | 1.052 | 1.068 | 1.085 | 1.193 | 1.352 | 1.557 | |||||
| Calcium chloride (CaCl2) | 1.028 | 1.050 | 1.078 | 1.110 | 1.143 | 1.319 | 1.564 | 1.930 | 3.467 | 8.997 | |||
| Potassium iodide (KI) | 0.997 | 0.991 | 0.986 | 0.981 | 0.976 | 0.946 | 0.925 | 0.910 | 0.892 | 0.897 | |||
| Cesium chloride (CsCl) | 0.997 | 0.992 | 0.988 | 0.984 | 0.980 | 0.966 | 0.953 | 0.939 | 0.922 | 0.934 | 0.981 | 1.120 | |
| Sucrose (C12H22O11) | 1.028 | 1.055 | 1.084 | 1.114 | 1.146 | 1.336 | 1.592 | 1.945 | 3.187 | 6.162 | 15.431 | 58.487 | 481.561 |
Substances of variable composition
| Substance | Viscosity (mPa·s) | Temperature (°C) | Reference |
|---|---|---|---|
| Whole milk | 2.12 | 20 | [39] |
| Blood | 2 - 9 | 37 | [40] |
| Olive oil | 56.2 | 26 | [39] |
| Canola oil | 46.2 | 30 | [39] |
| Sunflower oil | 48.8 | 26 | [39] |
| Honey | 2000-10000 | 20 | [41] |
| Ketchup[lower-alpha 1] | 5000-20000 | 25 | [42] |
| Peanut butter[lower-alpha 1] | 104-106 | [43] | |
| Pitch | 2.3×1011 | 10-30 (variable) | [44] |
- These materials are highly non-Newtonian.
Viscosities under nonstandard conditions
Gases
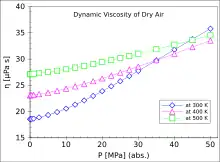
All values are given at 1 bar (approximately equal to atmospheric pressure).
| Substance | Chemical formula | Temperature (K) | Viscosity (μPa·s) |
|---|---|---|---|
| Air | 100 | 7.1 | |
| 200 | 13.3 | ||
| 300 | 18.5 | ||
| 400 | 23.1 | ||
| 500 | 27.1 | ||
| 600 | 30.8 | ||
| Ammonia | NH3 | 300 | 10.2 |
| 400 | 14.0 | ||
| 500 | 17.9 | ||
| 600 | 21.7 | ||
| Carbon dioxide | CO2 | 200 | 10.1 |
| 300 | 15.0 | ||
| 400 | 19.7 | ||
| 500 | 24.0 | ||
| 600 | 28.0 | ||
| Helium | He | 100 | 9.6 |
| 200 | 15.1 | ||
| 300 | 19.9 | ||
| 400 | 24.3 | ||
| 500 | 28.3 | ||
| 600 | 32.2 | ||
| Water vapor | H2O | 380 | 12.498 |
| 400 | 13.278 | ||
| 450 | 15.267 | ||
| 500 | 17.299 | ||
| 550 | 19.356 | ||
| 600 | 21.425 | ||
| 650 | 23.496 | ||
| 700 | 25.562 | ||
| 750 | 27.617 | ||
| 800 | 29.657 | ||
| 900 | 33.680 | ||
| 1000 | 37.615 | ||
| 1100 | 41.453 | ||
| 1200 | 45.192 |
Liquids (including liquid metals)
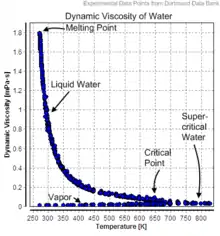
| Substance | Chemical formula | Temperature (°C) | Viscosity (mPa·s) |
|---|---|---|---|
| Mercury[45][46] | Hg | -30 | 1.958 |
| -20 | 1.856 | ||
| -10 | 1.766 | ||
| 0 | 1.686 | ||
| 10 | 1.615 | ||
| 20 | 1.552 | ||
| 25 | 1.526 | ||
| 30 | 1.495 | ||
| 50 | 1.402 | ||
| 75 | 1.312 | ||
| 100 | 1.245 | ||
| 126.85 | 1.187 | ||
| 226.85 | 1.020 | ||
| 326.85 | 0.921 | ||
| Ethanol | C2H6O | -25 | 3.26 |
| 0 | 1.786 | ||
| 25 | 1.074 | ||
| 50 | 0.694 | ||
| 75 | 0.476 | ||
| Bromine | Br2 | 0 | 1.252 |
| 25 | 0.944 | ||
| 50 | 0.746 | ||
| Water | H2O | 0.01 | 1.7911 |
| 10 | 1.3059 | ||
| 20 | 1.0016 | ||
| 25 | 0.89002 | ||
| 30 | 0.79722 | ||
| 40 | 0.65273 | ||
| 50 | 0.54652 | ||
| 60 | 0.46603 | ||
| 70 | 0.40355 | ||
| 80 | 0.35405 | ||
| 90 | 0.31417 | ||
| 99.606 | 0.28275 | ||
| Glycerol | C3H8O3 | 25 | 934 |
| 50 | 152 | ||
| 75 | 39.8 | ||
| 100 | 14.76 | ||
| Aluminum | Al | 700 | 1.24 |
| 800 | 1.04 | ||
| 900 | 0.90 | ||
| Gold | Au | 1100 | 5.130 |
| 1200 | 4.640 | ||
| 1300 | 4.240 | ||
| Copper | Cu | 1100 | 3.92 |
| 1200 | 3.34 | ||
| 1300 | 2.91 | ||
| 1400 | 2.58 | ||
| 1500 | 2.31 | ||
| 1600 | 2.10 | ||
| 1700 | 1.92 | ||
| Silver | Ag | 1300 | 3.75 |
| 1400 | 3.27 | ||
| 1500 | 2.91 | ||
| Iron | Fe | 1600 | 5.22 |
| 1700 | 4.41 | ||
| 1800 | 3.79 | ||
| 1900 | 3.31 | ||
| 2000 | 2.92 | ||
| 2100 | 2.60 |
In the following table, the temperature is given in kelvins.
| Substance | Chemical formula | Temperature (K) | Viscosity (mPa·s) |
|---|---|---|---|
| Gallium[46] | Ga | 400 | 1.158 |
| 500 | 0.915 | ||
| 600 | 0.783 | ||
| 700 | 0.700 | ||
| 800 | 0.643 | ||
| Zinc[46] | Zn | 700 | 3.737 |
| 800 | 2.883 | ||
| 900 | 2.356 | ||
| 1000 | 2.005 | ||
| 1100 | 1.756 | ||
| Cadmium[46] | Cd | 600 | 2.708 |
| 700 | 2.043 | ||
| 800 | 1.654 | ||
| 900 | 1.403 |
Solids
| Substance | Viscosity (Pa·s) | Temperature (°C) |
|---|---|---|
| granite[47] | 3×1019 - 6×1019 | 25 |
| asthenosphere[48] | 7.0×1019 | 900 |
| upper mantle[48] | 7×1020 – 1×1021 | 1300–3000 |
| lower mantle | 1×1021 – 2×1021 | 3000–4000 |
References
- Chapman, Sydney; Cowling, T.G. (1970), The Mathematical Theory of Non-Uniform Gases (3rd ed.), Cambridge University Press
- Kestin, J.; Ro, S. T.; Wakeham, W. A. (1972). "Viscosity of the Noble Gases in the Temperature Range 25–700°C". The Journal of Chemical Physics. 56 (8): 4119–4124. doi:10.1063/1.1677824. ISSN 0021-9606.
- Le Neindre, B.; Garrabos, Y.; Tufeu, R. (1989). "Thermal conductivity of dense noble gases". Physica A: Statistical Mechanics and Its Applications. 156 (1): 512–521. doi:10.1016/0378-4371(89)90137-4. ISSN 0378-4371.
- Ho, C. Y.; Powell, R. W.; Liley, P. E. (1972). "Thermal Conductivity of the Elements". Journal of Physical and Chemical Reference Data. 1 (2): 279–421. doi:10.1063/1.3253100. ISSN 0047-2689.
- Assael, M. J.; Kalyva, A. E.; Monogenidou, S. A.; Huber, M. L.; Perkins, R. A.; Friend, D. G.; May, E. F. (2018). "Reference Values and Reference Correlations for the Thermal Conductivity and Viscosity of Fluids". Journal of Physical and Chemical Reference Data. 47 (2): 021501. doi:10.1063/1.5036625. ISSN 0047-2689. PMC 6463310. PMID 30996494.
- Kestin, J.; Leidenfrost, W. (1959). "An absolute determination of the viscosity of eleven gases over a range of pressures". Physica. 25 (7–12): 1033–1062. doi:10.1016/0031-8914(59)90024-2. ISSN 0031-8914.
- Yaws, Carl L. (1997), Handbook Of Viscosity: Volume 4: Inorganic Compounds And Elements, Gulf Professional Publishing, ISBN 978-0123958501
- Kestin, J; Khalifa, H.E.; Wakeham, W.A. (1977). "The viscosity of five gaseous hydrocarbons". The Journal of Chemical Physics. 66 (3): 1132–1134. Bibcode:1977JChPh..66.1132K. doi:10.1063/1.434048.
- Titani, Toshizo (1930). "The viscosity of vapours of organic compounds. Part II". Bulletin of the Chemical Society of Japan. 5 (3): 98–108. doi:10.1246/bcsj.5.98.
- Miller, J.W. Jr.; Shah, P.N.; Yaws, C.L. (1976). "Correlation constants for chemical compounds". Chemical Engineering. 83 (25): 153–180. ISSN 0009-2460.
- Kestin, J.; Ro, S.T.; Wakeham, W.A. (1971). "Reference values of the viscosity of twelve gases at 25°C". Transactions of the Faraday Society. 67: 2308–2313. doi:10.1039/TF9716702308.
- Dunlop, Peter J. (1994). "Viscosities of a series of gaseous fluorocarbons at 25 °C". The Journal of Chemical Physics. 100 (4): 3149–3151. doi:10.1063/1.466405.
- Iwasaki, Hiroji; Takahashi, Mitsuo (1968). "Studies on the transport properties of fluids at high pressure". The Review of Physical Chemistry of Japan. 38 (1).
- "Database of the Thermophysical Properties of Gases Used in the Semiconductor Industry | NIST". Archived from the original on 2019-07-11. Retrieved 2019-07-11.
- Schäfer, Michael; Richter, Markus; Span, Roland (2015). "Measurements of the viscosity of carbon dioxide at temperatures from (253.15 to 473.15)K with pressures up to 1.2MPa". The Journal of Chemical Thermodynamics. 89: 7–15. doi:10.1016/j.jct.2015.04.015. ISSN 0021-9614.
- Kestin, J.; Ro, S. T.; Wakeham, W. A. (1982). "The Viscosity of Carbon-Monoxide and its Mixtures with Other Gases in the Temperature Range 25 - 200°C". Berichte der Bunsengesellschaft für physikalische Chemie. 86 (8): 753–760. doi:10.1002/bbpc.19820860816. ISSN 0005-9021.
- Pal, Arun K.; Bhattacharyya, P. K. (1969). "Viscosity of Binary Polar‐Gas Mixtures". The Journal of Chemical Physics. 51 (2): 828–831. doi:10.1063/1.1672075. ISSN 0021-9606.
- Takahashi, Mitsuo; Shibasaki-Kitakawa, Naomi; Yokoyama, Chiaki; Takahashi, Shinji (1996). "Viscosity of Gaseous Nitrous Oxide from 298.15 K to 398.15 K at Pressures up to 25 MPa". Journal of Chemical & Engineering Data. 41 (6): 1495–1498. doi:10.1021/je960060d. ISSN 0021-9568.
- Zarkova, L.; Hohm, U. (2002). "pVT–Second Virial Coefficients B(T), Viscosity eta(T), and Self-Diffusion rhoD(T) of the Gases: BF3, CF4, SiF4, CCl4, SiCl4, SF6, MoF6, WF6, UF6, C(CH3)4, and Si(CH3)4 Determined by Means of an Isotropic Temperature-Dependent Potential". Journal of Physical and Chemical Reference Data. 31 (1): 183–216. doi:10.1063/1.1433462. ISSN 0047-2689.
- chem.libretexts.org (11 March 2016). "Intermolecular Forces in Action: Surface Tension, Viscosity, and Capillary Action". chem.libretexts.org. Archived from the original on 2019-07-24. Retrieved 2019-07-24.
- CRC Handbook of Chemistry and Physics, 99th Edition (Internet Version 2018), John R. Rumble, ed., CRC Press/Taylor & Francis, Boca Raton, FL.
- Dymond, J. H.; Oye, H. A. (1994). "Viscosity of Selected Liquid n‐Alkanes". Journal of Physical and Chemical Reference Data. 23 (1): 41–53. doi:10.1063/1.555943. ISSN 0047-2689.
- Wu, Jianging; Nhaesi, Abdulghanni H.; Asfour, Abdul-Fattah A. (1999). "Viscosities of Eight Binary Liquidn-Alkane Systems at 293.15 K and 298.15 K". Journal of Chemical & Engineering Data. 44 (5): 990–993. doi:10.1021/je980291f. ISSN 0021-9568.
- Doolittle, Arthur K. (1951). "Studies in Newtonian Flow. II. The Dependence of the Viscosity of Liquids on Free‐Space". Journal of Applied Physics. 22 (12): 1471–1475. Bibcode:1951JAP....22.1471D. doi:10.1063/1.1699894. ISSN 0021-8979.
- Coursey, B. M.; Heric, E. L. (1971). "AApplication of the Congruence Principle to Viscosities of 1-Chloroalkane Binary Mixtures". Canadian Journal of Chemistry. 49 (16): 2631–2635. doi:10.1139/v71-437. ISSN 0008-4042.
- Wang, Jianji; Tian, Yong; Zhao, Yang; Zhuo, Kelei (2003). "A volumetric and viscosity study for the mixtures of 1-n-butyl-3-methylimidazolium tetrafluoroborate ionic liquid with acetonitrile, dichloromethane, 2-butanone and N, N ? dimethylformamide". Green Chemistry. 5 (5): 618. doi:10.1039/b303735e. ISSN 1463-9262.
- Reid, Robert C.; Prausnitz, John M.; Poling, Bruce E. (1987), The Properties of Gases and Liquids, McGraw-Hill Book Company, p. 442, ISBN 0-07-051799-1
- Venkatesulu, D.; Venkatesu, P.; Rao, M. V. Prabhakara (1997). "Viscosities and Densities of Trichloroethylene or Tetrachloroethylene with 2-Alkoxyethanols at 303.15 K and 313.15 K". Journal of Chemical & Engineering Data. 42 (2): 365–367. doi:10.1021/je960316f. ISSN 0021-9568.
- Nayak, Jyoti N.; Aralaguppi, Mrityunjaya I.; Aminabhavi, Tejraj M. (2003). "Density, Viscosity, Refractive Index, and Speed of Sound in the Binary Mixtures of Ethyl Chloroacetate + Cyclohexanone, + Chlorobenzene, + Bromobenzene, or + Benzyl Alcohol at (298.15, 303.15, and 308.15) K". Journal of Chemical & Engineering Data. 48 (3): 628–631. doi:10.1021/je0201828. ISSN 0021-9568.
- Cokelet, Giles R.; Hollander, Frederick J.; Smith, Joseph H. (1969). "Density and viscosity of mixtures of 1,1,2,2-tetrabromoethane and 1-bromododecane". Journal of Chemical & Engineering Data. 14 (4): 470–473. doi:10.1021/je60043a017. ISSN 0021-9568.
- Wright, Franklin J. (1961). "Influence of Temperature on Viscosity of Nonassociated Liquids". Journal of Chemical & Engineering Data. 6 (3): 454–456. doi:10.1021/je00103a035. ISSN 0021-9568.
- Sagdeev, D. I.; Fomina, M. G.; Mukhamedzyanov, G. Kh.; Abdulagatov, I. M. (2014). "Experimental Study and Correlation Models of the Density and Viscosity of 1-Hexene and 1-Heptene at Temperatures from (298 to 473) K and Pressures up to 245 MPa". Journal of Chemical & Engineering Data. 59 (4): 1105–1119. doi:10.1021/je401015e. ISSN 0021-9568.
- Petrino, P. J.; Gaston-Bonhomme, Y. H.; Chevalier, J. L. E. (1995). "Viscosity and Density of Binary Liquid Mixtures of Hydrocarbons, Esters, Ketones, and Normal Chloroalkanes". Journal of Chemical & Engineering Data. 40 (1): 136–140. doi:10.1021/je00017a031. ISSN 0021-9568.
- Segur, J. B.; Oberstar, H. E. (1951). "Viscosity of Glycerol and Its Aqueous Solutions". Industrial & Engineering Chemistry. 43 (9): 2117–2120. doi:10.1021/ie50501a040.
- Hetherington, G.; Robinson, P.L. (1956). "The Viscosities of Iodine Pentafluoride and Ditellurium Decafluoride". Journal of the Chemical Society (Resumed): 3681. doi:10.1039/jr9560003674. ISSN 0368-1769.
- Canosa, J.; Rodríguez, A.; Tojo, J. (1998). "Dynamic Viscosities of (Methyl Acetate or Methanol) with (Ethanol, 1-Propanol, 2-Propanol, 1-Butanol, and 2-Butanol) at 298.15 K". Journal of Chemical & Engineering Data. 43 (3): 417–421. doi:10.1021/je9702302. ISSN 0021-9568.
- Paez, Susana; Contreras, Martin (1989). "Densities and viscosities of binary mixtures of 1-propanol and 2-propanol with acetonitrile". Journal of Chemical & Engineering Data. 34 (4): 455–459. doi:10.1021/je00058a025. ISSN 0021-9568.
- Lal, Krishan; Tripathi, Neelima; Dubey, Gyan P. (2000). "Densities, Viscosities, and Refractive Indices of Binary Liquid Mixtures of Hexane, Decane, Hexadecane, and Squalane with Benzene at 298.15 K". Journal of Chemical & Engineering Data. 45 (5): 961–964. doi:10.1021/je000103x. ISSN 0021-9568.
- Fellows, P.J. (2009), Food Processing Technology: Principles and Practice (3rd ed.), Woodhead Publishing, ISBN 978-1845692162
- Pries, A. R.; Neuhaus, D.; Gaehtgens, P. (1992-12-01). "Blood viscosity in tube flow: dependence on diameter and hematocrit". American Journal of Physiology. Heart and Circulatory Physiology. 263 (6): H1770–H1778. doi:10.1152/ajpheart.1992.263.6.H1770. ISSN 0363-6135.
- Yanniotis, S.; Skaltsi, S.; Karaburnioti, S. (February 2006). "Effect of moisture content on the viscosity of honey at different temperatures". Journal of Food Engineering. 72 (4): 372–377. doi:10.1016/j.jfoodeng.2004.12.017.
- Koocheki, Arash; Ghandi, Amir; Razavi, Seyed M. A.; Mortazavi, Seyed Ali; Vasiljevic, Todor (2009), "The rheological properties of ketchup as a function of different hydrocolloids and temperature", International Journal of Food Science & Technology, 44 (3): 596–602, doi:10.1111/j.1365-2621.2008.01868.x
- Citerne, Guillaume P.; Carreau, Pierre J.; Moan, Michel (2001), "Rheological properties of peanut butter", Rheologica Acta, 40 (1): 86–96, doi:10.1007/s003970000120, S2CID 94555820
- Edgeworth, R; Dalton, B J; Parnell, T (1984), "The pitch drop experiment", European Journal of Physics, 5 (4): 198–200, Bibcode:1984EJPh....5..198E, doi:10.1088/0143-0807/5/4/003, S2CID 250769509
- Suhrmann, Von R.; Winter, E.-O. (1955), "Dichte- und Viskositätsmessungen an Quecksilber und hochverdünnten Kalium- und Cäsiumamalgamen vom Erstarrungspunkt bis + 30 C", Zeitschrift für Naturforschung, 10a (12): 985, doi:10.1515/zna-1955-1211, S2CID 97692836, archived from the original on 2020-02-15, retrieved 2021-10-17
- Assael, Marc J.; Armyra, Ivi J.; Brillo, Juergen; Stankus, Sergei V.; Wu, Jiangtao; Wakeham, William A. (2012), "Reference Data for the Density and Viscosity of Liquid Cadmium, Cobalt, Gallium, Indium, Mercury, Silicon, Thallium, and Zinc" (PDF), Journal of Physical and Chemical Reference Data, 41 (3): 033101, doi:10.1063/1.4729873, archived (PDF) from the original on 2021-10-17, retrieved 2019-12-12
- Kumagai, Naoichi; Sasajima, Sadao; Ito, Hidebumi (15 February 1978). "Long-term Creep of Rocks: Results with Large Specimens Obtained in about 20 Years and Those with Small Specimens in about 3 Years". Journal of the Society of Materials Science (Japan). 27 (293): 157–161. Archived from the original on 2011-05-21. Retrieved 2008-06-16.
- Fjeldskaar, W. (1994). "Viscosity and thickness of the asthenosphere detected from the Fennoscandian uplift". Earth and Planetary Science Letters. 126 (4): 399–410. Bibcode:1994E&PSL.126..399F. doi:10.1016/0012-821X(94)90120-1.