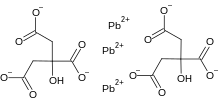
Lead citrate
Lead citrate is a compound of lead and citrate that is primarily used as an enhancer for heavy metal staining in electron microscopy.[2] This salt binds to osmium and uranyl acetate and enhances contrast in many cellular structures. Lead citrate is highly reactive with carbon dioxide.
 | |
| Names | |
|---|---|
| IUPAC name
2-hydroxypropane-1,2,3-tricarboxylate; lead(2+); trihydrate | |
| Other names
Lead citrate trihydrate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.402 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H10O14Pb3 | |
| Molar mass | 999.8 g·mol−1 |
| Appearance | White odorless powder or crystals |
| Density | 4.63 g/cm3 |
| Boiling point | 309.6 °C (589.3 °F; 582.8 K) |
| Soluble in water, slightly soluble in alcohol[1] | |
| Hazards | |
| GHS labelling: | |
 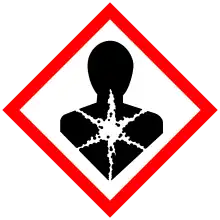 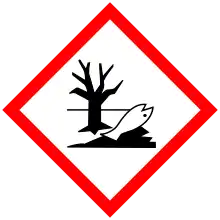 | |
| Danger | |
| H302, H332, H360, H373, H410 | |
| P201, P202, P260, P261, P264, P270, P271, P273, P281, P301+P312, P304+P312, P304+P340, P308+P313, P312, P314, P330, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Dale Perry (April 2016). Handbook of Inorganic Compounds. CRC Press. p. 225.
- Arun Sharma and Archana Sharma (2014). Chromosome Techniques: Theory and Practice. Butterworth-Heinemann. p. 285.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.