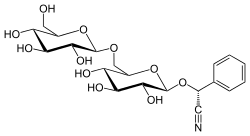
Amygdalin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2R)-[β-D-Glucopyranosyl-(1→6)-β-D-glucopyranosyloxy]phenylacetonitrile | |
| Systematic IUPAC name
(2R)-Phenyl{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-2-yl]oxy}acetonitrile | |
| Identifiers | |
3D model (JSmol) |
|
| 66856 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.045.372 |
| EC Number |
|
| MeSH | Amygdalin |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H27NO11 | |
| Molar mass | 457.429 |
| Melting point | 223-226 °C(lit.) |
| H2O: 0.1 g/mL hot, clear to very faintly turbid, colorless | |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302 | |
| P264, P270, P301+P312, P330, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | A6005 |
| Related compounds | |
Related compounds |
Vicianin, laetrile, prunasin, sambunigrin |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Amygdalin (from Ancient Greek: ἀμυγδαλή amygdalē 'almond') is a naturally occurring chemical compound found in many plants, most notably in the seeds (kernels) of apricots, bitter almonds, apples, peaches, cherries and plums, and in the roots of manioc.
Amygdalin is classified as a cyanogenic glycoside, because each amygdalin molecule includes a nitrile group, which can be released as the toxic cyanide anion by the action of a beta-glucosidase. Eating amygdalin will cause it to release cyanide in the human body, and may lead to cyanide poisoning.[1]
Since the early 1950s, both amygdalin and a chemical derivative named laetrile have been promoted as alternative cancer treatments, often under the misnomer vitamin B17 (neither amygdalin nor laetrile is a vitamin).[2] Scientific study has found them to not only be clinically ineffective in treating cancer, but also potentially toxic or lethal when taken by mouth due to cyanide poisoning.[3] The promotion of laetrile to treat cancer has been described in the medical literature as a canonical example of quackery,[4][5] and as "the slickest, most sophisticated, and certainly the most remunerative cancer quack promotion in medical history".[2]
Chemistry
Amygdalin is a cyanogenic glycoside derived from the aromatic amino acid phenylalanine. Amygdalin and prunasin are common among plants of the family Rosaceae, particularly the genus Prunus, Poaceae (grasses), Fabaceae (legumes), and in other food plants, including flaxseed and manioc. Within these plants, amygdalin and the enzymes necessary to hydrolyze it are stored in separate locations, and only mix as a result of tissue damage. This provides a natural defense system.[6]
Amygdalin is contained in stone fruit kernels, such as almonds, apricot (14 g/kg), peach (6.8 g/kg), and plum (4–17.5 g/kg depending on variety), and also in the seeds of the apple (3 g/kg).[7] Benzaldehyde released from amygdalin provides a bitter flavor. Because of a difference in a recessive gene called Sweet kernal [Sk], much less amygdalin is present in nonbitter (or sweet) almond than bitter almond.[8] In one study, bitter almond amygdalin concentrations ranged from 33 to 54 g/kg depending on variety; semibitter varieties averaged 1 g/kg and sweet varieties averaged 0.063 g/kg with significant variability based on variety and growing region.[9]
For one method of isolating amygdalin, the stones are removed from the fruit and cracked to obtain the kernels, which are dried in the sun or in ovens. The kernels are boiled in ethanol; on evaporation of the solution and the addition of diethyl ether, amygdalin is precipitated as minute white crystals.[10] Natural amygdalin has the (R)-configuration at the chiral phenyl center. Under mild basic conditions, this stereogenic center isomerizes; the (S)-epimer is called neoamygdalin. Although the synthesized version of amygdalin is the (R)-epimer, the stereogenic center attached to the nitrile and phenyl groups easily epimerizes if the manufacturer does not store the compound correctly.[11]
Amygdalin is hydrolyzed by intestinal β-glucosidase (emulsin)[12] and amygdalin beta-glucosidase (amygdalase) to give gentiobiose and L-mandelonitrile. Gentiobiose is further hydrolyzed to give glucose, whereas mandelonitrile (the cyanohydrin of benzaldehyde) decomposes to give benzaldehyde and hydrogen cyanide. Hydrogen cyanide in sufficient quantities (allowable daily intake: ~0.6 mg) causes cyanide poisoning which has a fatal oral dose range of 0.6–1.5 mg/kg of body weight.[13]
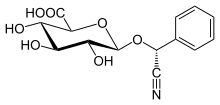
Laetrile
 | |
| Names | |
|---|---|
| IUPAC name
(2S,3S,4S,5R,6R)-6-[(R)-cyano(phenyl)methoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid | |
| Other names
L-mandelonitrile-β-D-glucuronide, Vitamin B17 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.045.372 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H15NO7 | |
| Molar mass | 309.2714 |
| Melting point | 214 to 216 °C (417 to 421 °F; 487 to 489 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Laetrile (patented 1961) is a simpler semisynthetic derivative of amygdalin. Laetrile is synthesized from amygdalin by hydrolysis. The usual preferred commercial source is from apricot kernels (Prunus armeniaca). The name is derived from the separate words "laevorotatory" and "mandelonitrile". Laevorotatory describes the stereochemistry of the molecule, while mandelonitrile refers to the portion of the molecule from which cyanide is released by decomposition.[14] A 500 mg laetrile tablet may contain between 2.5 and 25 mg of hydrogen cyanide.[15]
Like amygdalin, laetrile is hydrolyzed in the duodenum (alkaline) and in the intestine (enzymatically) to D-glucuronic acid and L-mandelonitrile; the latter hydrolyzes to benzaldehyde and hydrogen cyanide, that in sufficient quantities causes cyanide poisoning.[16]
Claims for laetrile were based on three different hypotheses:[17] The first hypothesis proposed that cancerous cells contained copious beta-glucosidases, which release HCN from laetrile via hydrolysis. Normal cells were reportedly unaffected, because they contained low concentrations of beta-glucosidases and high concentrations of rhodanese, which converts HCN to the less toxic thiocyanate. Later, however, it was shown that both cancerous and normal cells contain only trace amounts of beta-glucosidases and similar amounts of rhodanese.[17]
The second proposed that, after ingestion, amygdalin was hydrolyzed to mandelonitrile, transported intact to the liver and converted to a beta-glucuronide complex, which was then carried to the cancerous cells, hydrolyzed by beta-glucuronidases to release mandelonitrile and then HCN. Mandelonitrile, however, dissociates to benzaldehyde and hydrogen cyanide, and cannot be stabilized by glycosylation.[18]: 9
Finally, the third asserted that laetrile is the discovered vitamin B-17, and further suggests that cancer is a result of "B-17 deficiency". It postulated that regular dietary administration of this form of laetrile would, therefore, actually prevent all incidences of cancer. There is no evidence supporting this conjecture in the form of a physiologic process, nutritional requirement, or identification of any deficiency syndrome.[19] The term "vitamin B-17" is not recognized by Committee on Nomenclature of the American Institute of Nutrition Vitamins.[14] Ernst T. Krebs (not to be confused with Hans Adolf Krebs, the discoverer of the citric acid cycle) branded laetrile as a vitamin in order to have it classified as a nutritional supplement rather than as a pharmaceutical.[2]
Early usage
Amygdalin was first isolated in 1830 from bitter almond seeds (Prunus dulcis) by Pierre-Jean Robiquet and Antoine Boutron-Charlard.[20] Liebig and Wöhler found three hydrolysis products of amygdalin: sugar, benzaldehyde, and prussic acid (hydrogen cyanide, HCN).[21] Later research showed that sulfuric acid hydrolyzes it into D-glucose, benzaldehyde, and prussic acid; while hydrochloric acid gives mandelic acid, D-glucose, and ammonia.[22]
In 1845 amygdalin was used as a cancer treatment in Russia, and in the 1920s in the United States, but it was considered too poisonous.[23] In the 1950s, a purportedly non-toxic, synthetic form was patented for use as a meat preservative,[24] and later marketed as laetrile for cancer treatment.[23]
The U.S. Food and Drug Administration prohibited the interstate shipment of amygdalin and laetrile in 1977.[25][26] Thereafter, 27 U.S. states legalized the use of amygdalin within those states.[27]
Subsequent results
In a 1977 controlled, blinded trial, laetrile showed no more activity than placebo.[28]
Subsequently, laetrile was tested on 14 tumor systems without evidence of effectiveness. The Memorial Sloan–Kettering Cancer Center (MSKCC) concluded that "laetrile showed no beneficial effects."[28] Mistakes in an earlier MSKCC press release were highlighted by a group of laetrile proponents led by Ralph Moss, former public affairs official of MSKCC who had been fired following his appearance at a press conference accusing the hospital of covering up the benefits of laetrile.[29] These mistakes were considered scientifically inconsequential, but Nicholas Wade in Science stated that "even the appearance of a departure from strict objectivity is unfortunate."[28] The results from these studies were published all together.[30]
A 2015 systematic review from the Cochrane Collaboration found:
The claims that laetrile or amygdalin have beneficial effects for cancer patients are not currently supported by sound clinical data. There is a considerable risk of serious adverse effects from cyanide poisoning after laetrile or amygdalin, especially after oral ingestion. The risk–benefit balance of laetrile or amygdalin as a treatment for cancer is therefore unambiguously negative.[3]
The authors also recommended, on ethical and scientific grounds, that no further clinical research into laetrile or amygdalin be conducted.[3]
Given the lack of evidence, laetrile has not been approved by the U.S. Food and Drug Administration or the European Commission.
The U.S. National Institutes of Health evaluated the evidence separately and concluded that clinical trials of amygdalin showed little or no effect against cancer.[23] For example, a 1982 trial by the Mayo Clinic of 175 patients found that tumor size had increased in all but one patient.[31] The authors reported that "the hazards of amygdalin therapy were evidenced in several patients by symptoms of cyanide toxicity or by blood cyanide levels approaching the lethal range."
The study concluded "Patients exposed to this agent should be instructed about the danger of cyanide poisoning, and their blood cyanide levels should be carefully monitored. Amygdalin (Laetrile) is a toxic drug that is not effective as a cancer treatment".
Additionally, "No controlled clinical trials (trials that compare groups of patients who receive the new treatment to groups who do not) of laetrile have been reported."[23]
The side effects of laetrile treatment are the symptoms of cyanide poisoning. These symptoms include: nausea and vomiting, headache, dizziness, cherry red skin color, liver damage, abnormally low blood pressure, droopy upper eyelid, trouble walking due to damaged nerves, fever, mental confusion, coma, and death.
The European Food Safety Agency's Panel on Contaminants in the Food Chain has studied the potential toxicity of the amygdalin in apricot kernels. The Panel reported, "If consumers follow the recommendations of websites that promote consumption of apricot kernels, their exposure to cyanide will greatly exceed" the dose expected to be toxic. The Panel also reported that acute cyanide toxicity had occurred in adults who had consumed 20 or more kernels and that in children "five or more kernels appear to be toxic".[18]
Advocacy and legality of laetrile
Advocates for laetrile assert that there is a conspiracy between the US Food and Drug Administration, the pharmaceutical industry and the medical community, including the American Medical Association and the American Cancer Society, to exploit the American people, and especially cancer patients.[32]
Advocates of the use of laetrile have also changed the rationale for its use, first as a treatment of cancer, then as a vitamin, then as part of a "holistic" nutritional regimen, or as treatment for cancer pain, among others, none of which have any significant evidence supporting its use.[32]
Despite the lack of evidence for its use, laetrile developed a significant following due to its wide promotion as a "pain-free" treatment of cancer as an alternative to surgery and chemotherapy that have significant side effects. The use of laetrile led to a number of deaths.[32] The FDA and AMA crackdown, begun in the 1970s, effectively escalated prices on the black market, played into the conspiracy narrative and enabled unscrupulous profiteers to foster multimillion-dollar smuggling empires.[33]
Some American cancer patients have traveled to Mexico for treatment with the substance, for example at the Oasis of Hope Hospital in Tijuana.[34] The actor Steve McQueen died in Mexico following surgery to remove a stomach tumor, having previously undergone extended treatment for pleural mesothelioma (a cancer associated with asbestos exposure) under the care of William D. Kelley, a de-licensed dentist and orthodontist who claimed to have devised a cancer treatment involving pancreatic enzymes, 50 daily vitamins and minerals, frequent body shampoos, enemas, and a specific diet as well as laetrile.[35]
Laetrile advocates in the United States include Dean Burk, a former chief chemist of the National Cancer Institute cytochemistry laboratory,[36] and national arm wrestling champion Jason Vale, who falsely claimed that his kidney and pancreatic cancers were cured by eating apricot seeds. Vale was convicted in 2004 for, among other things, fraudulently marketing laetrile as a cancer cure.[37] The court also found that Vale had made at least $500,000 from his fraudulent sales of laetrile.[38]
In the 1970s, court cases in several states challenged the FDA's authority to restrict access to what they claimed are potentially lifesaving drugs. More than twenty states passed laws making the use of laetrile legal. After the unanimous Supreme Court ruling in United States v. Rutherford[39] which established that interstate transport of the compound was illegal, usage fell off dramatically.[14][40] The US Food and Drug Administration continues to seek jail sentences for vendors marketing laetrile for cancer treatment, calling it a "highly toxic product that has not shown any effect on treating cancer."[41]
In popular culture
The Law & Order episode "Second Opinion" is about a nutritional counselor named "Doctor" Haas giving patients laetrile as a cancer treatment for breast cancer as an alternative to getting a mastectomy.
References
- "Apricot kernels pose risk of cyanide poisoning". European Food Safety Authority. 27 April 2016.
A naturally-occurring compound called amygdalin is present in apricot kernels and converts to hydrogen cyanide after eating. Cyanide poisoning can cause nausea, fever, headaches, insomnia, thirst, lethargy, nervousness, joint and muscle various aches and pains, and falling blood pressure. In extreme cases it is fatal
- Lerner IJ (1981). "Laetrile: a lesson in cancer quackery". CA: A Cancer Journal for Clinicians. 31 (2): 91–5. doi:10.3322/canjclin.31.2.91. PMID 6781723. S2CID 28917628.
- Milazzo S, Horneber M (April 2015). "Laetrile treatment for cancer". The Cochrane Database of Systematic Reviews. 2018 (4): CD005476. doi:10.1002/14651858.CD005476.pub4. PMC 6513327. PMID 25918920.
- Lerner IJ (February 1984). "The whys of cancer quackery". Cancer. 53 (3 Suppl): 815–9. doi:10.1002/1097-0142(19840201)53:3+<815::AID-CNCR2820531334>3.0.CO;2-U. PMID 6362828. S2CID 36332694.
- Nightingale SL (1984). "Laetrile: the regulatory challenge of an unproven remedy". Public Health Reports. 99 (4): 333–8. PMC 1424606. PMID 6431478.
- Mora CA, Halter JG, Adler C, Hund A, Anders H, Yu K, Stark WJ (May 2016). "Application of the Prunus spp. Cyanide Seed Defense System onto Wheat: Reduced Insect Feeding and Field Growth Tests". Journal of Agricultural and Food Chemistry. 64 (18): 3501–7. doi:10.1021/acs.jafc.6b00438. PMID 27119432.
- Bolarinwa, Islamiyat F.; Orfila, Caroline; Morgan, Michael R.A. (2014). "Amygdalin content of seeds, kernels and food products commercially-available in the UK" (PDF). Food Chemistry. 152: 133–139. doi:10.1016/j.foodchem.2013.11.002. PMID 24444917. Archived (PDF) from the original on 9 October 2022.
- Sanchez-Perez, R.; Jorgensen, K.; Olsen, C. E.; Dicenta, F.; Moller, B. L. (2008). "Bitterness in Almonds". Plant Physiology. 146 (3): 1040–1052. doi:10.1104/pp.107.112979. PMC 2259050. PMID 18192442.
- Lee, Jihyun; Zhang, Gong; Wood, Elizabeth; Rogel Castillo, Cristian; Mitchell, Alyson E. (2013). "Quantification of Amygdalin in Nonbitter, Semibitter, and Bitter Almonds (Prunus dulcis) by UHPLC-(ESI)QqQ MS/MS". Journal of Agricultural and Food Chemistry. 61 (32): 7754–7759. doi:10.1021/jf402295u. PMID 23862656. S2CID 22497338.
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. Vol. 1 (11th ed.). Cambridge University Press. p. 900.
- Wahab MF, Breitbach ZS, Armstrong DW, Strattan R, Berthod A (October 2015). "Problems and Pitfalls in the Analysis of Amygdalin and Its Epimer". Journal of Agricultural and Food Chemistry. 63 (40): 8966–73. doi:10.1021/acs.jafc.5b03120. PMID 26431391.
- George Mann F, Charles Saunders B (1975). Practical Organic Chemistry (4th ed.). London: Longman. pp. 509–517. ISBN 9788125013808. Retrieved 1 February 2016.
- "Medical Management Guidelines (MMGs): Hydrogen Cyanide (HCN)". ATSDR. 21 October 2014. Retrieved 8 July 2019.
- Pdq Integrative, Alternative (15 March 2017). "Laetrile/Amygdalin (PDQ®)–Health Professional Version: General Information". cancer.gov. Complementary and Alternative Medicine for Health Professionals. National Cancer Institute. PMID 26389425. Retrieved 9 May 2017.
- Jerrold B. Leikin; Frank P. Paloucek, eds. (2008), "Laetrile", Poisoning and Toxicology Handbook (4th ed.), Informa, p. 950, ISBN 978-1-4200-4479-9
- Rietjens IM, Martena MJ, Boersma MG, Spiegelenberg W, Alink GM (February 2005). "Molecular mechanisms of toxicity of important food-borne phytotoxins". Molecular Nutrition & Food Research. 49 (2): 131–58. doi:10.1002/mnfr.200400078. PMID 15635687.
- Duke JA (2003). CRC Handbook of Medicinal Spices. CRC Press. pp. 261–262. ISBN 978-0-8493-1279-3.
- "Acute health risks related to the presence of cyanogenic glycosides in raw apricot kernels and products derived from raw apricot kernels". EFSA Journal. 14 (4). 2016. doi:10.2903/j.efsa.2016.4424.
- Greenberg DM (April 1975). "The vitamin fraud in cancer quackery". The Western Journal of Medicine. 122 (4): 345–8. PMC 1129741. PMID 1154776.
- "A chronology of significant historical developments in the biological sciences". Botany Online Internet Hypertextbook. University of Hamburg, Department of Biology. 18 August 2002. Archived from the original on 20 August 2007. Retrieved 6 August 2007.
- Wöhler, F.; Liebig, J. (1837). "Ueber die Bildung des Bittermandelöls". Annalen der Pharmacie. 22 (1): 1–24. doi:10.1002/jlac.18370220102. S2CID 96869201.
- Walker, J. W.; Krieble, V. K. (1909). "The hydrolysis of amygdalin by acids. Part I". Journal of the Chemical Society. 95 (11): 1369–77. doi:10.1039/CT9099501369.
- "Laetrile/Amygdalin". National Cancer Institute. 23 September 2005.
- US 2985664, E Krebs, Ernst T. & Krebs, Jr., Ernst T., "Hexuronic acid derivatives", published 23 May 1961
- Carpenter D (2010). Reputation and Power: Organizational Image and Pharmaceutical Regulation at the FDA. Princeton: Princeton University Press. Princeton: Princeton University Press. ISBN 978-0-691-14180-0.
- Kennedy D (1977). "Laetrile: The Commissioner's Decision" (PDF). Federal Register. Docket No. 77-22310.
- American Cancer Society (1991). "Unproven methods of cancer management. Laetrile". CA: A Cancer Journal for Clinicians. 41 (3): 187–92. doi:10.3322/canjclin.41.3.187. PMID 1902140. S2CID 5932239.
- Wade N (December 1977). "Laetrile at Sloan-Kettering: a question of ambiguity". Science. 198 (4323): 1231–4. Bibcode:1977Sci...198.1231W. doi:10.1126/science.198.4323.1231. PMID 17741690.
- Budiansky S (9 July 1995). "Cures or Quackery: How Senator Harkin shaped federal research on alternative medicine". U.S. News & World Report. Archived from the original on 3 September 2011. Retrieved 7 November 2009.
- Stock CC, Tarnowski GS, Schmid FA, Hutchison DJ, Teller MN (1978). "Antitumor tests of amygdalin in transplantable animal tumor systems". Journal of Surgical Oncology. 10 (2): 81–8. doi:10.1002/jso.2930100202. PMID 642516. S2CID 5896930.
Stock CC, Martin DS, Sugiura K, Fugmann RA, Mountain IM, Stockert E, Schmid FA, Tarnowski GS (1978). "Antitumor tests of amygdalin in spontaneous animal tumor systems". Journal of Surgical Oncology. 10 (2): 89–123. doi:10.1002/jso.2930100203. PMID 347176. S2CID 22185766. - "Laetrile (amygdalin, vitamin B17)". cancerhelp.org.uk. 30 August 2017.
- Editors of Consumer Reports Books (1980). "Laetrile: the Political Success of a Scientific Failure". Health Quackery. Vernon, New York: Consumers Union. pp. 16–40. ISBN 978-0-89043-014-9.
{{cite book}}:|last=has generic name (help) - Herbert, V (1979). "Laetrile: the cult of cyanide Promoting poison for profit". The American Journal of Clinical Nutrition. 32 (5): 1121–1158. doi:10.1093/ajcn/32.5.1121. PMID 219680.
- Moss RW (March 2005). "Patient perspectives: Tijuana cancer clinics in the post-NAFTA era". Integrative Cancer Therapies. 4 (1): 65–86. doi:10.1177/1534735404273918. PMID 15695477.
- Lerner BH (15 November 2005). "McQueen's Legacy of Laetrile". The New York Times. Retrieved 23 April 2010.
- "Dean Burk, 84, Noted Chemist At National Cancer Institute, Dies". The Washington Post. 9 October 1988. Archived from the original on 5 November 2012. Retrieved 14 January 2007.
- McWilliams BS (2005). Spam kings: the real story behind the high-rolling hucksters pushing porn, pills and @*#?% enlargements. Sebastopol, CA: O'Reilly. p. 237. ISBN 978-0-596-00732-4.
Jason Vale.
- "New York Man Sentenced to 63 Months for Selling Fake Cancer Cure". Medical News Today. 22 June 2004. Retrieved 8 July 2010.
- United States v. Rutherford, 442 U.S. 544 (United States Supreme Court 1979).
- Curran WJ (March 1980). "Law-medicine notes. Laetrile for the terminally ill: Supreme Court stops the nonsense". The New England Journal of Medicine. 302 (11): 619–21. doi:10.1056/NEJM198003133021108. PMID 7351911.
- "Lengthy Jail Sentence for Vendor of Laetrile – A Quack Medication to Treat Cancer Patients". FDA. 22 June 2004. Archived from the original on 10 July 2009.
