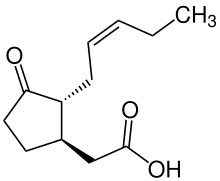
Jasmonic acid
Jasmonic acid (JA) is an organic compound found in several plants including jasmine. The molecule is a member of the jasmonate class of plant hormones. It is biosynthesized from linolenic acid by the octadecanoid pathway. It was first isolated in 1957 as the methyl ester of jasmonic acid by the Swiss chemist Édouard Demole and his colleagues.[1]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
{(1R,2R)-3-Oxo-2-[(2Z)-pent-2-en-1-yl]cyclopentyl}acetic acid | |
| Other names
Jasmonic acid (−)-Jasmonic acid JA (1R,2R)-3-Oxo-2-(2Z)-2-pentenylcyclopentylethanoic acid {(1R,2R)-3-Oxo-2-[(2Z)-2-penten-1-yl]cyclopentyl}acetic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H18O3 | |
| Molar mass | 210.27 g/mol |
| Density | 1.1 g/cm3 |
| Boiling point | 160 °C (320 °F; 433 K) at 0.7 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
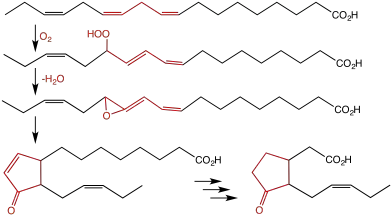
Biosynthesis
Its biosynthesis starts from the fatty acid linolenic acid, which is oxygenated by lipoxygenase (13-LOX), forming a hydroperoxide. This peroxide then cyclizes in the presence of allene oxide synthase to form an allene oxide. The rearrangement of allene oxide to form 12-oxophytodienoic acid is catalyzed by the enzyme allene oxide cyclase. A series of β-oxidations results in 7-isojasmonic acid. In the absence of enzyme, this isojasmonic acid isomerizes to jasmonic acid.[2]
Function
The major function of JA and its various metabolites is regulating plant responses to abiotic and biotic stresses as well as plant growth and development.[3] Regulated plant growth and development processes include growth inhibition, senescence, tendril coiling, flower development and leaf abscission. JA is also responsible for tuber formation in potatoes and yams. It has an important role in response to wounding of plants and systemic acquired resistance. The Dgl gene is responsible for maintaining levels of JA during usual conditions in Zea mays as well as the preliminary release of jasmonic acid shortly after being fed upon.[4] When plants are attacked by insects, they respond by releasing JA, which activates the expression of protease inhibitors, among many other anti-herbivore defense compounds. These protease inhibitors prevent proteolytic activity of the insects' digestive proteases or "salivary proteins",[5] thereby stopping them from acquiring the needed nitrogen in the protein for their own growth.[6] JA also activates the expression of Polyphenol oxidase which promotes the production of quinolines. These can interfere with the insect's enzyme production and decrease the nutrition content of the ingested plant.[7]
JA may have a role in pest control.[8] Indeed, JA has been considered as a seed treatment in order to stimulate the natural anti-pest defenses of the plants that germinate from the treated seeds. In this application jasmonates are sprayed onto plants that have already started growing.[9] These applications stimulate the production of protease inhibitor in the plant.[10] This production of protease inhibitor can protect the plant from insects, decreasing infestation rates and physical damage sustained due to herbivores.[11] However, due to its antagonistic relationship with salicylic acid (an important signal in pathogen defense) in some plant species, it may result in an increased susceptibility to viral agents and other pathogens.[12] In Zea mays, salicylic acid and JA are mediated by NPR1 (nonexpressor of pathogenesis-related genes1), which is essential in preventing herbivores from exploiting this antagonistic system.[13] Armyworms (Spodoptera caterpillars), through unknown mechanisms, are able to increase the activity of the salicylic acid pathway in maize, resulting in the depression of JA synthesis, but thanks to NPR1 mediation, JA levels aren't decreased by a significant amount.[13]
Derivatives
Jasmonic acid is also converted to a variety of derivatives including the ester methyl jasmonate. This conversion is catalyzed by the jasmonic acid carboxyl methyltransferase enzyme.[14] It can also be conjugated to amino acids in some biological contexts. Decarboxylation affords the related fragrance jasmone.
References
- Demole, E.; Lederer, E.; Mercier, D. (1962). "Isolement et determination de la structure du jasmonate de methyle, constituent odorant characteristique de l'essence de jasmin" [Isolation and determination of the structure of methyl jasmonate, the aromatic constituent characteristic of jasmine essential oil]. Helvetica Chimica Acta (in French). 45 (2): 675–685. doi:10.1002/hlca.19620450233.
- Chapuis, Christian (December 2011). "The chemistry and creative legacy of methyl jasmonate and hedione". Perfumer & Flavorist. 36: 36–48.
- Dewick, Paul (2009). Medicinal Natural Products: A Biosynthetic Approach. United Kingdom: John Wiley & Sons. pp. 42–53. ISBN 978-0-470-74168-9.
- Delker, C.; Stenzel, I.; Hause, B.; Miersch, O.; Feussner, I.; Wasternack, C. (2006). "Jasmonate Biosynthesis in Arabidopsis thaliana – Enzymes, Products, Regulation". Plant Biology. 8 (3): 297–306. doi:10.1055/s-2006-923935. PMID 16807821.
- Gális, I.; Gaquerel, E.; Pandey, S. P.; Baldwin, I. N. T. (2009). "Molecular mechanisms underlying plant memory in JA-mediated defence responses". Plant, Cell & Environment. 32 (6): 617–627. doi:10.1111/j.1365-3040.2008.01862.x. PMID 18657055.
- Lutz, Diana (2012). "Key part of plants' rapid response system revealed". Washington University in St. Louis.
- Zavala, J. A.; Patankar, A. G.; Gase, K.; Hui, D.; Baldwin, I. T. (2004). "Manipulation of Endogenous Trypsin Proteinase Inhibitor Production in Nicotiana attenuata Demonstrates Their Function as Antiherbivore Defenses". Plant Physiology. 134 (3): 1181–1190. doi:10.1104/pp.103.035634. PMC 389942. PMID 14976235.
- The Effects of Bacterial and Jasmonic Acid Treatments on Insects of Canola. 2008.
- "Success for plants' pest control". BBC News. 2008-10-07. Retrieved 2010-05-05.
- Worrall, D.; Holroyd, G. H.; Moore, J. P.; Glowacz, M.; Croft, P.; Taylor, J. E.; Paul, N. D.; Roberts, M. R. (2012). "Treating seeds with activators of plant defence generates long-lasting priming of resistance to pests and pathogens" (PDF). New Phytologist. 193 (3): 770–778. doi:10.1111/j.1469-8137.2011.03987.x. PMID 22142268.
- Farmer, E. E.; Johnson, R. R.; Ryan, C. A. (March 1992). "Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic Acid". Plant Physiology. 98 (3): 995–1002. doi:10.1104/pp.98.3.995. ISSN 0032-0889. PMC 1080300. PMID 16668777.
- Fouad, Hany Ahmed; El-Gepaly, Hosam Mohamed Khalil Hammam; Fouad, Osama Ahmed (2016-08-26). "Nanosilica and jasmonic acid as alternative methods for control Tuta absoluta (Meyrick) in tomato crop under field conditions". Archives of Phytopathology and Plant Protection. 49 (13–14): 362–370. doi:10.1080/03235408.2016.1219446. ISSN 0323-5408. S2CID 89119004.
- Lyons, R.; Manners, J. M.; Kazan, K. (2013). "Jasmonate biosynthesis and signaling in monocots: A comparative overview". Plant Cell Reports. 32 (6): 815–27. doi:10.1007/s00299-013-1400-y. PMID 23455708. S2CID 10778582.
- Ballaré, Carlos L. (2011). "Jasmonate-induced defenses: A tale of intelligence, collaborators and rascals". Trends in Plant Science. 16 (5): 249–57. doi:10.1016/j.tplants.2010.12.001. PMID 21216178.
- Seo, H.-S.; Song, J.-T.; Cheong, J.-J.; Lee, Y.-H.; Lee, Y.-W.; Hwang, I.; Lee, J.-S.; Choi, Y.-D. (2001-04-10). "Jasmonic acid carboxyl methyltransferase: a key enzyme for jasmonate-regulated plant responses". Proceedings of the National Academy of Sciences. 98 (8): 4788–4793. Bibcode:2001PNAS...98.4788S. doi:10.1073/pnas.081557298. ISSN 0027-8424. PMC 31912. PMID 11287667.
- Sankawa, Ushio; Barton, Derek H. R.; Nakanishi, Koji; Meth-Cohn, Otto, eds. (1999). Comprehensive Natural Products Chemistry: Polyketides and Other Secondary Metabolites Including Fatty Acids and Their Derivatives. Pergamon Press. ISBN 978-0-08-043153-6.