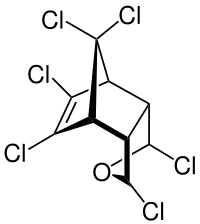
Isobenzan
Isobenzan (telodrin) is a highly toxic organochloride insecticide. It was produced only in the period from 1958 to 1965 and its use has been since discontinued.[1] It is a persistent organic pollutant that can remain in soil for 2 to 7 years, and the biological half-life of isobenzan in human blood is estimated to be about 2.8 years.[1]
 | |
| Names | |
|---|---|
| IUPAC name
1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran | |
| Other names
Telodrin; 1,3,4,5,6,7,8,8-Octachloro-4,7-methylene-3a,4,7,7a-tetrahydro-isobenzofuran | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.497 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2761 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H4Cl8O | |
| Molar mass | 411.73 g·mol−1 |
| Appearance | Whitish to light brown crystalline powder |
| Density | 1.87 g/cm3 |
| Melting point | 121.3 °C (250.3 °F; 394.4 K) |
| Practically insoluble | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H300, H310, H320, H361, H370, H372, H410 | |
| P201, P202, P260, P262, P264, P270, P273, P280, P281, P301+P310, P302+P350, P305+P351+P338, P307+P311, P308+P313, P310, P314, P321, P322, P330, P337+P313, P361, P363, P391, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[3]
References
- Isobenzan, International Programme on Chemical Safety
- Isobenzan at Sigma-Aldrich
- 40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities (PDF) (Report) (July 1, 2008 ed.). Government Printing Office. Archived from the original (PDF) on February 25, 2012. Retrieved October 29, 2011.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
