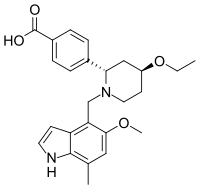
Iptacopan
Iptacopan (LNP023) is a drug developed by Novartis designed to treat paroxysmal nocturnal hemoglobinuria (PNH), a disease in which the innate immune system destroys red blood cells. It is the first drug that selectively inhibits factor B, the active component of the complement's C3 and C5 convertases.[1] In contrast to other PNH treatments like eculizumab, iptacopan is a small molecule.
 | |
| Clinical data | |
|---|---|
| Other names | LNP023 |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C25H30N2O4 |
| Molar mass | 422.525 g·mol−1 |
| 3D model (JSmol) | |
| |
In a clinical study with twelve participants, iptacopan as a single drug led to the normalization of hemolytic markers in most patients, and no serious adverse events occurred during the 12-week study.[1] [2]
Iptacopan is also investigated as a drug in other complement-mediated diseases, like age-related macular degeneration and some types of glomerulopathies.[3]
Sources
- Jang JH, Wong L, Ko BS, Yoon SS, Li K, Baltcheva I, et al. (August 2022). "Iptacopan monotherapy in patients with paroxysmal nocturnal hemoglobinuria: a 2-cohort open-label proof-of-concept study". Blood Advances. 6 (15): 4450–4460. doi:10.1182/bloodadvances.2022006960. PMC 9636331. PMID 35561315.
- "Novartis Phase III APPOINT-PNH trial shows investigational oral monotherapy iptacopan improves hemoglobin to near-normal levels, leading to transfusion independence in all treatment-naïve PNH patients". Novartis. Retrieved 2023-09-06.
- Schubart A, Anderson K, Mainolfi N, Sellner H, Ehara T, Adams CM, et al. (April 2019). "Small-molecule factor B inhibitor for the treatment of complement-mediated diseases". Proceedings of the National Academy of Sciences of the United States of America. 116 (16): 7926–7931. Bibcode:2019PNAS..116.7926S. doi:10.1073/pnas.1820892116. PMC 6475383. PMID 30926668.