Bisulfide
Bisulfide (or bisulphide in British English) is an inorganic anion with the chemical formula HS− (also written as SH−). It contributes no color to bisulfide salts, and its salts may have a distinctive putrid smell. It is a strong base. Bisulfide solutions are corrosive and attack the skin.
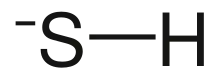 | |
 | |
| Names | |
|---|---|
| Systematic IUPAC name
sulfanide (rarely used, not common) | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| 24766 | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| HS− | |
| Molar mass | 33.07 g·mol−1 |
| Conjugate acid | Hydrogen sulfide |
| Conjugate base | Sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It is an important chemical reagent and an industrial chemical, mainly used in paper pulp industry (Kraft process), textiles, synthetic flavors, coloring brasses, and iron control.
Properties
A variety of salts are known, including sodium hydrosulfide and potassium hydrosulfide. Ammonium hydrosulfide, a component of "stink bombs" has not been isolated as a pure solid. Some compounds described as salts of the sulfide dianion contain primarily hydrosulfide. For example, the hydrated form of sodium sulfide, nominally with the formula Na2S · 9 H2O, is better described as NaSH · NaOH · 8 H2O.
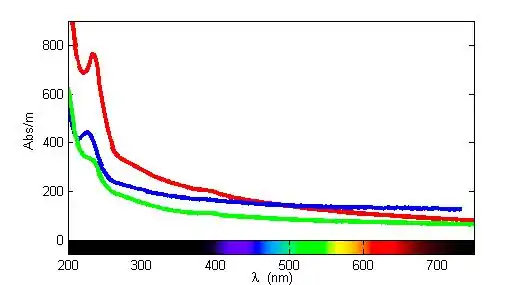
Aqueous bisulfide absorbs light at around 230 nm in the UV–visible spectrum.[1] Using this approach, bisulfide has been detected in the ocean[2][3] and in sewage.[4] Bisulfide should not be confused with the disulfide dianion, S2−2, or −S–S−.
Basicity
The bisulfide anion can accept a proton:
-
(1)
Because of its affinity to accept a proton (H+), bisulfide has a basic character. In aqueous solution, it has a corresponding pKa value of 6.9. Its conjugate acid is hydrogen sulfide (H2S). However, bisulfide's basicity stems from its behavior as an Arrhenius base. A solution containing spectator-only counter ions, has a basic pH according to the following acid-base reaction:
-
(2)
Chemical reactions
Upon treatment with an acid, bisulfide converts to hydrogen sulfide. With strong acids, it can be doubly protonated to give H
3S+
. Oxidation of bisulfide gives sulfate. When strongly heated, bisulfide salts decompose to produce sulfide salts and hydrogen sulfide.
-
2 HS− → H2S + S2−
(3)
Biochemistry
At physiological pH, hydrogen sulfide is usually fully ionized to bisulfide (HS−). Therefore, in biochemical settings, "hydrogen sulfide" is often used to mean, bisulfide. Hydrosulfide has been identified as the third gasotransmitter along with nitric oxide and carbon monoxide.[5]
Other derivatives
SH− is a soft anionic ligand that forms complexes with most metal ions. Examples include [Au(SH)2]− and (C5H5)2Ti(SH)2, derived from gold(I) chloride and titanocene dichloride, respectively.[6]
Safety
Bisulfide salts are corrosive, strongly alkaline and release toxic hydrogen sulfide upon acidification.
References
- Goldhaber, M.B.; Kaplan, I.R. (1975), "Apparent dissociation constants of hydrogen sulfide in chloride solutions", Marine Chemistry, 3 (1): 83–104, doi:10.1016/0304-4203(75)90016-X
- Johnson, K.S.; Coletti, L.S. (2001), "In situ ultraviolet spectrophotometry for high resolution and long-term monitoring of nitrate, bromide and bisulfide in the ocean.", Deep-Sea Research, 49 (7): 1291–1305, Bibcode:2002DSRI...49.1291J, doi:10.1016/s0967-0637(02)00020-1
- Guenther, E.A.; Johnson, K.S.; Coale, K.H. (2001), "Direct ultraviolet spectrophotometric determination of total sulfide and iodide in natural waters", Analytical Chemistry, 73 (14): 3481–3487, doi:10.1021/ac0013812, PMID 11476251
- Sutherland-Stacey, L.; Corrie, S.; Neethling, A.; Johnson, I.; Gutierrez, O.; Dexter, R.; Yuan, Z.; Keller, J.; Hamilton, G. (2007), "Continuous measurement of dissolved sulfide in sewer systems", Water Science and Technology
- J. W. Pavlik, B. C. Noll, A. G. Oliver, C. E. Schulz, W. R. Scheidt, “Hydrosulfide (HS−) Coordination in Iron Porphyrinates”, Inorganic Chemistry, 2010, vol. 49(3), 1017-1026.
- Peruzzini, M.; de los Rios, I. & Romerosa, A. (2001), "Coordination Chemistry of Transition Metals with Hydrogen Chalcogenide and Hydrogen Chalcogenido Ligands", Progress in Inorganic Chemistry, 49: 169–543, doi:10.1002/9780470166512.ch3, ISBN 978-0-470-16651-2