H2-CBD
H2CBD (DiHydroCBD, partially hydrogenated CBD) are cannabinoids that were first synthesized by the Todd group in 1940 by catalytic hydrogenation of cannabidiol.[1]
 | |
| Names | |
|---|---|
| IUPAC name
2'-isopropyl-5'-methyl-4-pentyl-1',2',3',4'-tetrahydro-[1,1'-biphenyl]-2,6-diol or 2-(5-methyl-2-(prop-1-en-2-yl)cyclohexyl)-5-pentylbenzene-1,3-diol | |
| Other names
Hydrogenated CBD, HCBD, HCBD, DiHydroCBD, DiHydroCannabidiol | |
| Identifiers | |
| Properties | |
| C21H32O2 | |
| Molar mass | 316.485 g·mol−1 |
| Related compounds | |
Related compounds |
H4-CBD |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
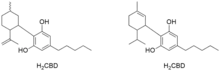
The term "H2CBD" can refer to two different chemical compounds that differ by the site of hydrogenation, either saturated on the cyclohexenyl ring (i.e. 1,2-dihydrocannabidiol using the older terpenoid numbering scheme) or saturated on the isopropenyl side group, which is known as 8,9-dihydrocannabidiol.
H2CBD, H4-CBD, and 8,9-dihydrocannabidiol have all been referred to as "hydrogenated CBD" which may cause confusion.
Pharmacology
In 2006, it was discovered that 8,9-dihydrocannabidiol binds very weakly to the CB1 receptor with a binding affinity higher than 1 µM, but has potential anti-inflammatory effects independent of its cannabinoid receptor action.[2]
See also
References
- Jacob, A.; Todd, A. R. (1940). "119. Cannabis indica. Part II. Isolation of cannabidiol from Egyptian hashish. Observations on the structure of cannabinol". J. Chem. Soc. 119: 649–653. doi:10.1039/jr9400000649.
- Ben-Shabat, Shimon; Hanuš, Lumír O.; Katzavian, Galia; Gallily, Ruth (February 2006). "New Cannabidiol Derivatives: Synthesis, Binding to Cannabinoid Receptor, and Evaluation of Their Antiinflammatory Activity". Journal of Medicinal Chemistry. 49 (3): 1113–1117. doi:10.1021/jm050709m. PMID 16451075.