Glutarimide
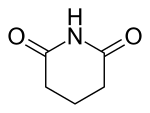
Glutarimide is the organic compound with the formula (CH2)3(CO)2NH. It is a white solid. The compound forms upon dehydration of the amide of glutaric acid.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Piperidine-2,6-dione | |
| Other names
2,6-Diketopiperidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.038 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H7NO2 | |
| Molar mass | 113.11 g/mol |
| Melting point | 155-157 °C[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Glutarimide is sometimes called 2,6-piperidinedione. It is the core of a variety of drugs, including thalidomide, a medication used to treat multiple myeloma[3] and leprosy,[4] and cycloheximide, a potent inhibitor of protein synthesis.[5]
References
- Glutarimide - Sigma-Aldrich
- Paris, G.; Berlinguet, L.; Gaudry, R.; English, Jr., J.; Dayan, J. E. (1957). "Glutaric Acid and Glutarimide". Organic Syntheses. 37: 47. doi:10.15227/orgsyn.037.0047.
- "A to Z List of Cancer Drugs: Thalidomide". National Cancer Institute. Retrieved 20 September 2021.
- Stolberg SG (17 July 1998). "Thalidomide Approved to Treat Leprosy, With Other Uses Seen". New York Times. Retrieved 20 September 2021.
- Sisler, Hugh D.; Siegel, Malcolm R. (1967). "Cycloheximide and Other Glutarimide Antibiotics". Mechanism of Action. pp. 283–307. doi:10.1007/978-3-642-46051-7_21. ISBN 978-3-642-46053-1.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.