Epanorin
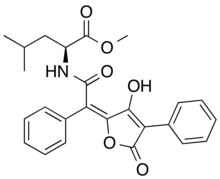
Epanorin is a lichen secondary metabolite with the molecular formula C25H25NO6.[2] Epanorin inhibits the proliferation of MCF-7 cancer cells.[2]
 | |
| Names | |
|---|---|
| IUPAC name
(1R,2R,13S,15R,16R,23R)-7,9,21-triazahexacyclo[11.9.1.11,15.02,7.09,23.016,21]tetracosane | |
| Other names
CHEBI:144243 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
PubChem CID |
|
| |
| |
| Properties | |
| C25H25NO6 | |
| Molar mass | 435.476 g·mol−1 |
| Melting point | 162–163 °C (324–325 °F; 435–436 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- "Epanorin,18463-10-0 - LookChemical.com". www.lookchemical.com.
- Palacios-Moreno, Juan; Rubio, Cecilia; Quilhot, Wanda; Cavieres, M. Fernanda; de la Peña, Eduardo; Quiñones, Natalia V.; Díaz, Hugo; Carrión, Flavio; Henríquez-Roldán, Carlos F.; Weinstein-Oppenheimer, Caroline R. (December 2019). "Epanorin, a lichen secondary metabolite, inhibits proliferation of MCF-7 breast cancer cells". Biological Research. 52 (1): 55. doi:10.1186/s40659-019-0261-4.
Further reading
- Issues in Life Sciences: Botany and Plant Biology Research: 2011 Edition. ScholarlyEditions. 9 January 2012. ISBN 978-1-4649-6343-8.
- Der Stoffwechsel Sekundärer Pflanzenstoffe / The Metabolism of Secondary Plant Products. Springer Science & Business Media. 11 November 2013. p. 572. ISBN 978-3-662-26784-4.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.