Enoyl CoA isomerase
Enoyl-CoA-(∆) isomerase (EC 5.3.3.8, also known as dodecenoyl-CoA-(∆) isomerase, 3,2-trans-enoyl-CoA isomerase, ∆3(cis),∆2(trans)-enoyl-CoA isomerase, or acetylene-allene isomerase,[1] is an enzyme that catalyzes the conversion of cis- or trans-double bonds of coenzyme A (CoA) bound fatty acids at gamma-carbon (position 3) to trans double bonds at beta-carbon (position 2) as below:
| Δ3-Δ2-Enoyl-CoA isomerase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
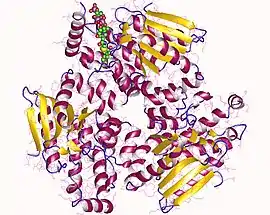 3,2-trans-enoyl-CoA isomerase trimer, Human | |||||||||
| Identifiers | |||||||||
| EC no. | 5.3.3.8 | ||||||||
| CAS no. | 62213-29-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
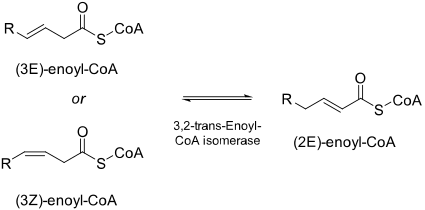
This enzyme has an important role in the metabolism of unsaturated fatty acids in beta oxidation.
Mechanism
Enoyl-CoA isomerase is involved in the beta-oxidation, one of the most frequently used pathways in fatty acid degradation, of unsaturated fatty acids with double bonds at odd-numbered carbon positions.[2] It does so by shifting the position of the double bonds in the acyl-CoA intermediates and converting 3-cis or trans-enoyl-CoA to 2-trans-enoyl-CoA. Since the key step in the degradation of fatty acids with double bonds at even-numbered carbon positions also produces 3-trans-enoyl-CoA in mammals and yeasts, enoyl-CoA isomerase is technically required for their metabolism as well.[3] The reaction mechanism is detailed in figure 1,[4] and the base that initiates the isomerization and NH groups that stabilize the intermediate are located on the active site of enoyl-coA isomerase.
As it functions in the step immediately preceding the actual beta-oxidation and forms a double bond extending from the beta-carbon (position 2), enoyl-CoA isomerase is involved in both the NADPH-dependent and NADPH-independent pathways of beta-oxidation.[5] The double bond serves as the target of oxidation and carbon-to-carbon bond cleavage, thereby shortening the fatty acid chain.
Sub-classification
Enoyl-CoA isomerases can be categorized into three classes:
- monofunctional mitochondrial
- monofunctional peroxisomal
- multifunctional
The monofunctional mitochondrial and peroxisomal enzymes are found in the mitochondria and peroxisomes of eukaryotes, respectively. The multifunctional enzymes are found in bacteria and in the peroxisomes of some eukaryotes, but they serve two functions: the N-terminal domain works the same as the other classes of enoyl-CoA isomerases and the C-terminal domain works as a dehydrogenase, specifically, to 3-hydroxyactyl-CoA.[4] There are two divisions among the mitochondrial enoyl Co-A isomerase: short-chain and long-chain [4].[6] In an immunoblot, antibodies were run against all enoyl CoA isomerase. However, two of these isomerases had antibody attachment: the short chain isomerase and the peroxisomal multifunctional enzyme.[6] There was one enzyme which did not have binding specificity to this antibody: mitochondrial long-chain isomerase. Long-chain isomerase was found when it eluted at a lower potassium phosphate concentration in the gradient.[6][7] Thus, the discovery of three sub-classes of enoyl CoA isomerase was made.
Although all three classes of enzymes have the same function, there is little overlap among their amino acid sequences. For example, only 40 out of 302 amino acid sequences (13%) are the same between monofunctional peroxisomal and mitochondrial enzymes in humans.[4] In fact, in mammals, the peroxisomal enzyme has an extra N-terminal domain that is not present in the mitochondrial counterpart.[8] Also, it has been found to be a subunit of the peroxisomal trifunctional enzyme (pTFE) and contributes only to minor cleavages of the fatty acid chain. In that sense, for many higher organisms, the mitochondrial enzyme is essential for deriving maximum energy from lipids and fueling muscles.[9]
Mitochondria (both short- and long-chain) of rat liver contain more than one enoyl Co-A isomerase.[10] To further support the idea that short- and long-chain isomerases elute at different concentration of potassium phosphate concentration, they do not share similar primary polypeptide structure, hence they must not be evolutionarily related.[6][11] Peroxisomes of plants and of rat liver are very different in the way they operate. Despite their primary structure similarities, there are differences among the different specimen. To begin with, the peroxisomes of rat liver are a multifunctional enzyme including enoyl-CoA isomerase, enoyl-CoA hydratase, and L-(−)-3-hydroxyacyl-CoA dehydrogenase.[12] Three different enzymes reside on this entity (multifunctional protein) allowing this enzyme to perform isomerization, hydration, and dehydration.[13][14] Isomerase activity on the multifunctional enzyme occurs at the amino-terminal catalytic half of the protein along with the hydratase activity.[15] The dehydrogenase activity of enoyl-CoA occurs in the carboxyl-terminal.[15] Upon further investigation of the CoA binding site on the amino-terminal half of the multifunctional protein, the CoA substrate is not transferred through the aqueous phase from the isomerization phase to the site of hydration or does not have a bulk phase.[11][16] This removes the need for a substrate transferring enzyme.[17] On the other hand, the cotyledons convert long-chain 3-trans-enoyl-CoA, long-chain 3-cis-enoyl-CoA, and short-chain 3-cis-enoyl-CoA species into their 2- trans-enoyl-CoA respective forms.[13] As previously mentioned, plant enoyl-CoA isomerase exclusively forms the 2-trans isomer as product. It does not act on 4-cis-enoyl-CoA species or 2-trans- 4-trans-dienoyl-CoA species.[13] In comparing the products of the plant peroxisome and the multifunctional enzyme of rat liver, the plant has no hydratase activity.[13] The Plant form did not form a 2-cis-isomer (from enoyl-CoA hydratase) or D- or L- 3 hydroxy derivative (L-(−)-3-hydroxyacyl-CoA dehydrogenase): products of multifunctional enzyme of rat liver.[13] The turnover rates of these the two sub divisions of peroxisomes are very different. The Kcat/Km ratio in cotyledons is 10^6 M-1s-1 which outperforms the ratio .07 * 10^6 M-1s-1.[13] Due to a high turnover rate, the plant peroxisomes contain a lesser amount of enoyl-CoA isomerase than their counterparts in the rat liver.[13]
In rat liver, mitochondrial enoyl CoA isomerase and peroxisomal enoyl CoA isomerase embedded in the multifunctional enzyme have similarities in the primary structure sequence.[15] When comparing the amino-terminal half of E. coli against the amino-terminal half of rat liver, there were primary and secondary structure similarities towards the middle of the amino-terminal end.[15] This conserved region must be important for structure and function of this specific enzyme since showing equally in both E. coli and rat liver.[15][18]
Structure
All classes of enoyl-CoA isomerases belong to a family of enzymes, the hydratase/isomerase or crotonase superfamily, and when examined with x-ray crystallography, exhibit a common structural feature of the family, the N-terminal core with a spiral fold composed of four turns, each turn consisting of two beta-sheets and one alpha-helix.[19]
In enoyl-CoA isomerase, the two beta-sheets are part of the catalytic site, since the NH groups of residues following the beta-sheets attach to the carbonyl oxygen of the acyl-CoA intermediate. The formation of this oxyanion hole stabilizes the transition state of the enzyme-catalyzed reaction.[4]
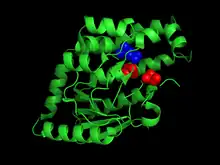
Moreover, a glutamate residue located next to body cavities filled with water molecules and lined with hydrophobic or apolar side chains has also been identified as a part of the catalytic site. In its deprotonated form, the glutamate can act as a base and remove a proton from the acyl-CoA intermediate. The body cavities aid in rearranging the glutamate side chain to retain the proton and later deliver it back to the acyl-CoA, on a different carbon position.[4]
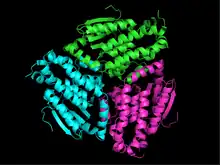
The NH-containing residues have been identified as Ala70 and Leu126 and the glutamate as Glu158 in peroxisomal enzymes in a yeast species, Saccharomyces cerevisiae. Their relative locations on the enzyme can be compared in figure 2.[4]
The enzymes of the hydratase/isomerase or crotonase superfamily are typically trimeric disks dimerized into hexamers. The wide range of their substrate-enzyme specificity derives from the variations in the distances between the trimeric disks and their orientation.[20] However, the human mitochondrial enoyl-CoA isomerase is a trimer and orients the fatty acid tail in a completely different direction from that seen in the hexamers.[8] The trimeric disk of peroxisomal enzymes in Saccharomyces cerevisiae is displayed in figure 3.[20]
History
Enoyl-CoA isomerase was first identified and purified from rat liver mitochondria in the 1960s and 1970s via gel filtration and ion exchange chromatography.[21] Since then, all classes of enoyl-CoA isomerase, mitochondrial, peroxisomal and multifunctional, have been identified in different organisms, including more mammals, plants, and unicellular organisms.
By 1994, using the rat enoyl-CoA isomerase cDNA as a hybridization probe, human enoyl-CoA isomerase cDNA could be sequenced and cloned.[2] In the same year, the protein itself was isolated, not by affinity to rat antibody or cDNA probes,[3] but by copurification with a transferase, human glutathione S-transferases.[22]
In the attempts to examine the human enoyl-CoA isomerase in detail, the mitochondrial enzyme in the mammalian liver was identified as a potential biological marker for metabolic diseases due to its elevated levels in defective cells, and linked defects in fatty acid beta-oxidation to human diseases,[22] to be specified in the next section.
Clinical significance
In humans, defects in the beta-oxidation mechanism result in hypoketotic hyperglycemia, a symptom of starvation, due to the inefficient utilization of fatty acids as a primary source of energy.[9] The metabolic disease was found to be on a genetic level: rats without the genes for enoyl-CoA isomerase also displayed high blood glucose level. Moreover, a biological marker for this condition may have been identified as the urine of these rats included high concentrations of medium chain unsaturated dicarboxylic acids, a condition called dicarboxylic aciduria.[9]
More recent studies link hepatitis C virus (HCV) infection to defects in fatty acid degradation, specifically, to that in enoyl-CoA isomerase.[23] HCV is the leading cause of chronic hepatitis, cirrhosis, and liver cancer, and more than 180 million people are affected globally.[24] Due to the prolonged latency of the virus and no existing cures to rid the virus specifically,[25] HCV is a serious problem that is causing more deaths than HIV/AIDS in the United States,[26] but its threat still do not receive adequate attention. The need for a HCV-specific treatment is essential, and according to John Ward, the director of the CDC Hepatitis Division, it can save up to 120,000 lives.[26]
According to protein profiling in the human liver biopsies of HCV patients, a correlation was initially discovered between dysfunctional mitochondrial processes, which include beta-oxidation, and HCV.[27] As a matter of fact, lipids play an important role in the replication cycle of HCV, and in the "in vivo" samples from HCV patients, many lipids were found in abundance to aid HCV in virus uptake, RNA replication, and secretion from host cells. Enzymes that regulate fatty acid metabolism, including enoyl-CoA isomerase, were also similarly upregulated.[23] Gene silencing techniques revealed that enoyl-CoA isomerase is essential in HCV RNA replication, and opened ways to stop HCV infection on an intracellular level.[23]
References
- "ENZYME entry 5.3.3.8". Retrieved 1 March 2012.
- Janssen U, Fink T, Lichter P, Stoffel W (September 1994). "Human mitochondrial 3,2-trans-enoyl-CoA isomerase (DCI): gene structure and localization to chromosome 16p13.3". Genomics. 23 (1): 223–8. doi:10.1006/geno.1994.1480. PMID 7829074.
- Kilponen JM, Häyrinen HM, Rehn M, Hiltunen JK (May 1994). "cDNA cloning and amino acid sequence of human mitochondrial delta 3 delta 2-enoyl-CoA isomerase: comparison of the human enzyme with its rat counterpart, mitochondrial short-chain isomerase". Biochemical Journal. 300 (1): 1–5. doi:10.1042/bj3000001. PMC 1138113. PMID 8198519.
- Mursula AM, van Aalten DM, Hiltunen JK, Wierenga RK (June 2001). "The crystal structure of delta(3)-delta(2)-enoyl-CoA isomerase". J. Mol. Biol. 309 (4): 845–53. doi:10.1006/jmbi.2001.4671. PMID 11399063. S2CID 69172923.
- Luo MJ, Smeland TE, Shoukry K, Schulz H (January 1994). "Delta 3,5, delta 2,4-dienoyl-CoA isomerase from rat liver mitochondria. Purification and characterization of a new enzyme involved in the beta-oxidation of unsaturated fatty acids". J. Biol. Chem. 269 (4): 2384–8. doi:10.1016/S0021-9258(17)41957-0. PMID 8300563.
- Kilponen, J. M.; Palosaari, P. M.; Hiltunen, J. K. (1990). "Occurrence of a long-chain delta 3,delta 2-enoyl-CoA isomerase in rat liver". Biochemical Journal. 269 (1): 223–226. doi:10.1042/bj2690223. PMC 1131556. PMID 2375752.
- Brian V. Geisbrecht; Dai Zhu; Kerstin Schulz; Katja Nau; James C. Morrell; Michael Geraghty; Horst Schulz; Ralf Erdmann; Stephen J. Gould (1998). "Molecular Characterization of Saccharomyces cerevisiae delta3, delta2-Enoyl-CoA Isomerase". Journal of Biological Chemistry. 273 (50): 33184–33191. doi:10.1074/jbc.273.50.33184. PMID 9837886.
- Partanen ST, Novikov DK, Popov AN, Mursula AM, Hiltunen JK, Wierenga RK (September 2004). "The 1.3 A crystal structure of human mitochondrial Delta3-Delta2-enoyl-CoA isomerase shows a novel mode of binding for the fatty acyl group". J. Mol. Biol. 342 (4): 1197–208. doi:10.1016/j.jmb.2004.07.039. PMID 15351645.
- Janssen U, Stoffel W (May 2002). "Disruption of mitochondrial beta -oxidation of unsaturated fatty acids in the 3,2-trans-enoyl-CoA isomerase-deficient mouse". J. Biol. Chem. 277 (22): 19579–84. doi:10.1074/jbc.M110993200. PMID 11916962.
- Palosaari P.M.; Hiltunen, J. K. (1991). "Purification and characterization of a plant peroxisomal delta2 ,delta3-enoyl-CoA isomerase acting on 3-cis-enoyl-CoA and 3-trans-enoyl-CoA" (PDF). Eur. J. Biochem. 196 (3): 699–705. doi:10.1111/j.1432-1033.1991.tb15868.x. PMID 2013292.
- Ptiivi M. Palosaari; Johanna M. Kilponen; Raija T. Sormunenn; Ilmo E. Hassine; J. Kalervo Hiltunen (1989). "Characterization of the Mitochondrial Isonzyme in the Rat" (PDF). Journal of Biological Chemistry. 265 (6): 3347–3353. PMID 2154476.
- Gerhard Muller-Newen; Uwe Janssen; Wilhelm Stoffel (1995). "Enoyl-CoA hydratase and isomerase form a superfamily with a common active site glutamate residue". Eur. J. Biochem. 228 (1): 68–73. doi:10.1111/j.1432-1033.1995.tb20230.x. PMID 7883013.
- Palosaari PM, Kilponen JM, Sormunen RT, Hassinen E, Hiltunen JK (1990). "Delta 3,delta 2-enoyl-CoA isomerases. Characterization of the mitochondrial isoenzyme in the rat". J. Biol. Chem. 265 (6): 3347–53. doi:10.1016/S0021-9258(19)39773-X. PMID 2154476.
- Dongyan Zhang; Wenfeng Yu; Brian V. Geisbrecht; Stephen J. Gould; Howard Sprecher; Horst Schulz (2002). "Functional Characterization of delta3,delta2-Enoyl-CoA Isomerases from Rat Liver". Journal of Biological Chemistry. 277 (11): 9127–9132. doi:10.1074/jbc.m112228200. PMID 11781327.
- Paivi M. Palosaari; Mauno Vihinen; Pekka 1. Mantsalag; Stefan E.H. Alexsonll; Taina Pihlajaniemi; J. Kalervo Hiltunen (1991). "Amino Acid Sequence Similarities of the Mitochondrial Short Chain delta3,delta2-Enoyl-CoA Isomerase and Peroxisomal Multifunctional delta3,delta2- Enoyl-CoA Isomerase, 2-Enoyl-CoA Hydratase, 3-Hydroxyacyl-CoA Dehydrogenase Enzyme in Rat Liver" (PDF). Journal of Biological Chemistry. 266 (17): 10750–10753. doi:10.1016/S0021-9258(18)99081-2. PMID 2040594.
- Patricia C. Babbitt; George L. Kenyon (1992). "Ancestry of the 4-Chlorobenzoate Dehalogenase: Analysis of Amino Acid Sequence Identities among Families of Acyl: Adenyl Ligases, Enoyl-CoA Hydratases/Isomerases, and Acyl-CoA Thioesterases". Biochemistry. 31 (24): 5594–5604. doi:10.1021/bi00139a024. PMID 1351742.
- Anu M. Mursula; Daan M. F. van Aalten; J. Kalervo Hiltunen; Rik K. Wierenga (2001). "The Crystal Structure of delta3-delta2-Enoyl-CoA Isomerase". Molecular Biology. 309 (4): 845–853. doi:10.1006/jmbi.2001.4671. PMID 11399063. S2CID 69172923.
- Aner Gurvitz; Anu M. Mursula; Andreas Firzinger; Barbara Hamilton; Seppo H. Kilpela ̈ inen; Andreas Hartig; Helmut Ruis; J. Kalervo Hiltunen; Hanspeter Rottensteiner (1998). "Peroxisomal delta3-cis-delta2-trans-Enoyl-CoA Isomerase Encoded by ECI1 Is Required for Growth of the Yeast Saccharomyces cerevisiae on Unsaturated Fatty Acids". Journal of Biological Chemistry. 273 (47): 31366–31374. doi:10.1074/jbc.273.47.31366. PMID 9813046.
- Gurvitz A, Mursula AM, Firzinger A, et al. (November 1998). "Peroxisomal Delta3-cis-Delta2-trans-enoyl-CoA isomerase encoded by ECI1 is required for growth of the yeast Saccharomyces cerevisiae on unsaturated fatty acids". J. Biol. Chem. 273 (47): 31366–74. doi:10.1074/jbc.273.47.31366. PMID 9813046.
- Mursula AM, Hiltunen JK, Wierenga RK (January 2004). "Structural studies on delta(3)-delta(2)-enoyl-CoA isomerase: the variable mode of assembly of the trimeric disks of the crotonase superfamily". FEBS Lett. 557 (1–3): 81–7. doi:10.1016/S0014-5793(03)01450-9. PMID 14741345.
- Stoffel W, Grol M (December 1978). "Purification and properties of 3-cis-2-trans-enoyl-CoA isomerase (dodecenoyl-CoA delta-isomerase) from rat liver mitochondria". Hoppe-Seyler's Z. Physiol. Chem. 359 (12): 1777–82. doi:10.1515/bchm2.1978.359.2.1777. PMID 738702.
- Takahashi Y, Hirata Y, Burstein Y, Listowsky I (December 1994). "Delta 3, delta 2-enoyl-CoA isomerase is the protein that copurifies with human glutathione S-transferases from S-hexylglutathione affinity matrices". Biochemical Journal. 304 (3): 849–52. doi:10.1042/bj3040849. PMC 1137411. PMID 7818490.
- Rasmussen AL, Diamond DL, McDermott JE, et al. (November 2011). "Systems virology identifies a mitochondrial fatty acid oxidation enzyme, dodecenoyl coenzyme A delta isomerase, required for hepatitis C virus replication and likely pathogenesis". J. Virol. 85 (22): 11646–54. doi:10.1128/JVI.05605-11. PMC 3209311. PMID 21917952.
- Rosen, Hugo R. (June 2011). "Chronic Hepatitis C Infection". The New England Journal of Medicine. 364 (25): 2429–2438. doi:10.1056/NEJMcp1006613. PMID 21696309. S2CID 19755395.
- Amemiya F, Maekawa S, Itakura Y, et al. (February 2008). "Targeting lipid metabolism in the treatment of hepatitis C virus infection". J. Infect. Dis. 197 (3): 361–70. doi:10.1086/525287. PMID 18248300.
- "Hepatitis C Kills More Americans Than HIV/AIDS". Voice of America, Health. 27 February 2012. Retrieved 3 March 2012.
- Diamond DL, Jacobs JM, Paeper B, et al. (September 2007). "Proteomic profiling of human liver biopsies: hepatitis C virus-induced fibrosis and mitochondrial dysfunction". Hepatology. 46 (3): 649–57. doi:10.1002/hep.21751. PMID 17654742.