Dimethyl carbate
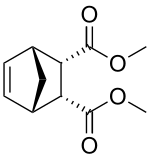
Dimethyl carbate is an insect repellent. It can be prepared by the Diels–Alder reaction of dimethyl maleate and cyclopentadiene.[2]
 | |
| Names | |
|---|---|
| IUPAC name
Dimethyl (1R,2S,3R,4S)-bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate | |
| Other names
Dimethyl cis-5-norbornene-2,3-dicarboxylate; Dimalone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C11H14O4 | |
| Molar mass | 210.229 g·mol−1 |
| Density | 1.4852 g/cm3[1] |
| Melting point | 38 °C (100 °F; 311 K)[1] |
| Pharmacology | |
| P03BX05 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Merck Index, 11th Edition, 3230
- Inukai, Takashi; Kojima, Takeshi (1966). "Aluminum chloride catalyzed diene condensation. II. Stronger adherence to the Alder endo rule". Journal of Organic Chemistry. 31: 2032–2033. doi:10.1021/jo01344a543. ISSN 0022-3263.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.