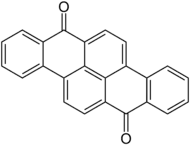
Dibenzpyrenequinone
Dibenzpyrenequinone is a synthetic vat dye. It is a bright yellow solid.[1] It can be produced by cyclization of 1,5-dibenzoylnaphthalene. Dibenzpyrenequinone is a precursor to Vat Orange 1.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Dibenzo[c,pqr]tetraphene-7,14-dione | |
| Other names
Vat yellow 4 Golden Yellow GK Dibenzochrysenedione Tyrian Yellow I-GOK C.I. 59100 Dibenzo[b,def]chrysene-7,14-dione 3,4:8,9-dibenzopyrene-5,10-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.459 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C24H12O2 | |
| Molar mass | 332.358 g·mol−1 |
| Appearance | yellow solid |
| Density | 1.418g/cm3 |
| Melting point | 385 °C (725 °F; 658 K) |
| Boiling point | 606.7 °C (1,124.1 °F; 879.9 K) at 760 mmHg |
| insoluble | |
| -250.3·10−6 cm3/mol | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Possible carcinogen |
| Flash point | 219.93 °C (427.87 °F; 493.08 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
See also
- Dibromoanthanthrone,Vat Orange 3
References
- Zherebtsov, D. A.; Nayfert, S. A.; Polozov, M. A.; Zhivulin, D. E.; Zhivulin, V. E.; Stash, A. I.; Chen, Yu-Sheng; Merzlov, S. V.; Bartashevich, E. V.; Avdin, V. V.; Hsu, Hua Shu; Guo, Feng Wei; Sakthidharan, C. P. (2018). "The Structure and Properties of 2,3-7,8-Dibenzpyrene-1,6-Quinone". Crystallography Reports. 63 (7): 1110–1115. Bibcode:2018CryRp..63.1110Z. doi:10.1134/s1063774518070283.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.