Citramalic acid
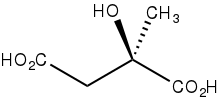
Citramalic acid is the organic compound with the formula HO2CCH2C(CH3)(OH)CO2H. A chiral compound, it is related structurally to malic acid.
 | |
| Names | |
|---|---|
| Other names
(R)-2-hydroxy-2-methylbutanedioic acid, 2-methylmalic acid, D-(−)-2-methylmalic acid, (R)-2-hydroxy-2-methylsuccinic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.154.104 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H8O5 | |
| Molar mass | 148.114 g·mol−1 |
| Appearance | white solid |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis and reactions
Citramalic acid is the hydrated derivative of mesaconic acid, The hydration is catalyzed by mesaconyl-C4-CoA hydratase:
- HO2CCH=C(CH3)CO2H + H2O → HO2CCH2C(CH3)(OH)CO2H
The same conversion can be achieved in vitro.[1]
The enzyme (S)-citramalyl-CoA lyase converts citramalyl-CoA to acetyl-CoA and pyruvate.[2]
References
- Eck, Richard; Simon, Helmut (1994). "Preparation of Both Enantiomers of Malic and Ctramalic Acid and Other Hydroxysuccinic Acid Derivatives by Stereospecific Hydrations of cis or trans 2-Butene-1,4-dioic Acids with Resting Cells of Clostridium formicoaceticum". Tetrahedron. 50 (48): 13641–13654. doi:10.1016/S0040-4020(01)85678-7.
- Berg, Ivan A. (2011). "Ecological Aspects of the Distribution of Different Autotrophic CO2 Fixation Pathways". Applied and Environmental Microbiology. 77 (6): 1925–1936. Bibcode:2011ApEnM..77.1925B. doi:10.1128/AEM.02473-10. PMC 3067309. PMID 21216907.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.