Calcium aluminates
Calcium aluminates are a range of materials[2] obtained by heating calcium oxide and aluminium oxide together at high temperatures. They are encountered in the manufacture of refractories and cements.
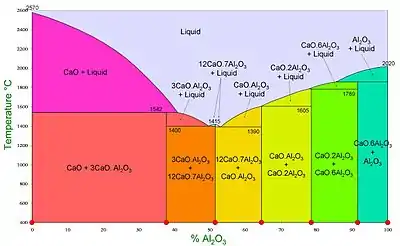
Calcium aluminates phase diagram.
The stable phases shown in the phase diagram (formed at atmospheric pressure under an atmosphere of normal humidity) are:
- Tricalcium aluminate, 3CaO·Al2O3 (C3A)
- Dodecacalcium hepta-aluminate, 12CaO·7Al2O3 (C12A7) (once known as mayenite[3])
- Monocalcium aluminate, CaO·Al2O3 (CA) (occurring in nature as krotite and dmitryivanovite - two polymorphs[4])
- Monocalcium dialuminate, CaO·2Al2O3 (CA2) (occurring in nature as grossite [5])
- Monocalcium hexa-aluminate, CaO·6Al2O3 (CA6) (occurring in nature as hibonite, a representative of magnetoplumbite group[6])
In addition, other phases include:
- Dicalcium aluminate, 2CaO·Al2O3 (C2A), which exists only at pressures above 2500 MPa.[7] The crystal is orthorhombic, with density 3480 kg·m−3. The natural dicalcium aluminate, brownmillerite, may form at normal pressure but elevated temperature in pyrometamorphic zones, e.g., in burning coal-mining heaps.[8]
- Pentacalcium trialuminate, 5CaO·3Al2O3 (C5A3), forms only under an anhydrous and oxygen free atmosphere. The crystal is orthorhombic, with a density of 3067 kg·m−3. It reacts rapidly with water.
- Tetracalcium trialuminate, 4CaO·3Al2O3 (C4A3), is a metastable phase formed by dehydrating 4CaO·3Al2O3·3H2O (C4A3H3).
Hydration reaction
In contrast to Portland cements, calcium aluminates do not release calcium hydroxide (Ca(OH)2, portlandite or lime) during their hydration.
See also
- Calcium aluminate cements
- Cement
- Cement chemist notation (CCN)
in which the following abbreviations for calcium and aluminium oxides are defined as: - Hydrocalumite
- Mayenite
- Ye'elimite, C4A3S̅, a rare natural anhydrous calcium sulfoaluminate
References
- Hosono, H.; Tanabe, K.; Takayama-Muromachi, E.; Kageyama, H.; Yamanaka, S.; Kumakura, H.; Nohara, M.; Hiramatsu, H.; Fujitsu, S. (2015). "Exploration of new superconductors and functional materials, and fabrication of superconducting tapes and wires of iron pnictides". Science and Technology of Advanced Materials. 16 (3): 033503. arXiv:1505.02240. Bibcode:2015STAdM..16c3503H. doi:10.1088/1468-6996/16/3/033503. PMC 5099821. PMID 27877784.
- Taylor H.F.W (1990) Cement Chemistry, Academic Press, ISBN 0-12-683900-X, pp. 34–38.
- "Mayenite Supergroup".
- "Krotite".
- "Grossite".
- "Hibonite".
- Taylor H.F.W (1990) Cement Chemistry, Academic Press, ISBN 0-12-683900-X, pp. 28, 29.
- "Brownmillerite".
Further reading
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.