CETP inhibitor
A CETP inhibitor is a member of a class of drugs that inhibit cholesterylester transfer protein (CETP).[1][2][3][4] They are intended to reduce the risk of atherosclerosis (a cardiovascular disease) by improving blood lipid levels. At least three medications within this class have failed to demonstrate a beneficial effect.[5]
| CETP inhibitor | |
|---|---|
| Drug class | |
| Class identifiers | |
| Use | None as of 2017 |
| Biological target | Cholesterylester transfer protein |
| Legal status | |
| In Wikidata | |
Types
These drugs have generally failed in clinical trials, either causing a marked increase in deaths (torcetrapib), or having no meaningful clinical improvement despite HDL increases (dalcetrapib, evacetrapib).
Failed:
- Torcetrapib, failed in 2006 due to excess deaths in Phase III clinical trials.
- Dalcetrapib, development halted in May 2012 when Phase III trials failed to show clinically meaningful efficacy.[6]
- Evacetrapib, development discontinued in 2015 due to insufficient efficacy.[7]
Succeeded:
- Anacetrapib. In 2017, the REVEAL trial based on more than 30,000 participants showed a modest benefit of the addition of anacetrapib to statin therapy.[8] Despite the successful trial, Merck halted the development of the drug.[9] Discontinued in 2017.[10]
- Obicetrapib (TA-8995, AMG-899), Phase II results reported in 2015,[11]
Mechanism
Drugs in this class substantially increase HDL cholesterol, lower LDL cholesterol, and enhance reverse cholesterol transport.
CETP inhibitors inhibit cholesterylester transfer protein (CETP), which normally transfers cholesterol from HDL cholesterol to very low density or low density lipoproteins (VLDL or LDL). Inhibition of this process results in higher HDL levels and reduces LDL levels.[12] CETP inhibitors do not reduce rates of mortality, heart attack, or stroke in patients already taking a statin.[13]
Pharmacogenomics
In 2015, a pharmacogenomic sub-study of the dal-OUTCOMES clinical trial on 5,749 individuals identified a genetic variant in the ADCY9 gene which modulates response to dalcetrapib. In patients with the rs1967309 'AA' genotype, there was a significant reduction in the rate of cardiovascular events in the dalcetrapib arm whereas non-carriers were at increased risk.[14] Beginning in 2015, the efficacy of dalcetrapib in the genetic sub-population was being investigated in the dal-GenE trial.[15]
Chemical structures
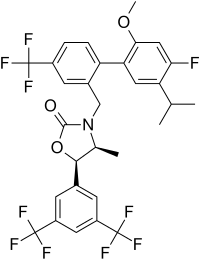


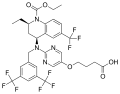 Obicetrapib
Obicetrapib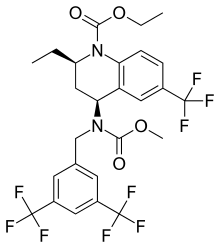
References
- Tall AR (March 2007). "CETP inhibitors to increase HDL cholesterol levels". N. Engl. J. Med. 356 (13): 1364–6. doi:10.1056/NEJMe078029. PMID 17387130.
- Joy TR, Hegele RA (August 2008). "The failure of torcetrapib: what have we learned?". Br. J. Pharmacol. 154 (7): 1379–81. doi:10.1038/bjp.2008.248. PMC 2492099. PMID 18536741.
- Rennings AJ, Stalenhoef AF (October 2008). "JTT-705: is there still future for a CETP inhibitor after torcetrapib?". Expert Opin Investig Drugs. 17 (10): 1589–97. doi:10.1517/13543784.17.10.1589. PMID 18808319.
- Carmen Drahl (February 2012). "The Cholesterol Bet". Chemical & Engineering News. 90 (8): 13–20.
- Filippatos, TD; Kei, A; Elisaf, MS (29 September 2017). "Anacetrapib, a New CETP Inhibitor: The New Tool for the Management of Dyslipidemias?". Diseases. 5 (4): 21. doi:10.3390/diseases5040021. PMC 5750532. PMID 28961179.
- Larry Husten (May 2012). "Roche Terminates Development Of CETP Inhibitor Dalcetrapib". Forbes.
- "Lilly to Discontinue Development of Evacetrapib for High-Risk Atherosclerotic Cardiovascular Disease". Oct 12, 2015.
- Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ (September 2017). "Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease". The New England Journal of Medicine. 377 (13): 1217–1227. doi:10.1056/NEJMoa1706444. PMID 28847206.
- "Merck Provides Update on Anacetrapib Development Program". Merck.com. October 11, 2017. Retrieved January 4, 2018.
- "Notable drug failures in 2017". Chemical & Engineering News. 95 (48). December 4, 2017.
- Hovingh, G Kees; Kastelein, John J P; Van Deventer, Sander J H; Round, Patrick; Ford, John; Saleheen, Danish; Rader, Daniel J; Brewer, H Bryan; Barter, Philip J (2015). "Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): A randomised, double-blind, placebo-controlled phase 2 trial". The Lancet. 386 (9992): 452–60. doi:10.1016/S0140-6736(15)60158-1. hdl:1887/117246. PMID 26047975.
- Barkowski RS, Frishman WH (May 2018). "HDL metabolism and CETP inhibition". Cardiol Rev. 16 (3): 154–62. doi:10.1097/CRD.0b013e31816a3b60. PMID 18414186.
- Keene D, Price C, Shun-Shin MJ, Francis DP (July 2014). "Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients". BMJ (Clinical Research Ed.). 349: g4379. doi:10.1136/bmj.g4379. PMC 4103514. PMID 25038074.
- Tardif, Jean-Claude; Rhéaume, Eric; Lemieux Perreault, Louis-Philippe; Grégoire, Jean C.; Feroz Zada, Yassamin; Asselin, Géraldine; Provost, Sylvie; Barhdadi, Amina; Rhainds, David (2015-04-01). "Pharmacogenomic determinants of the cardiovascular effects of dalcetrapib". Circulation: Cardiovascular Genetics. 8 (2): 372–382. doi:10.1161/CIRCGENETICS.114.000663. ISSN 1942-3268. PMID 25583994.
- "Effect of Dalcetrapib vs Placebo on CV Risk in a Genetically Defined Population With a Recent ACS - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-12-02.