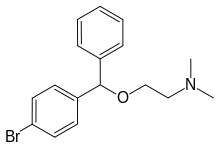
Bromazine
Bromazine, sold under the brand names Ambodryl, Ambrodil, and Deserol among others, also known as bromodiphenhydramine, is an antihistamine and anticholinergic medication of the ethanolamine class.[1][2][3][4][5] It is an analogue of diphenhydramine with a bromine substitution on one of the phenyl rings.[1][2]
 | |
| Clinical data | |
|---|---|
| Trade names | Ambodryl, Ambrodil, Deserol |
| Other names | Bromodiphenhydramine; Bromdiphenhydramine |
| MedlinePlus | a682065 |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | High |
| Protein binding | 96% |
| Metabolism | Mostly hepatic (CYP-mediated), also renal |
| Elimination half-life | 1 to 4 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.003.854 |
| Chemical and physical data | |
| Formula | C17H20BrNO |
| Molar mass | 334.257 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Synthesis
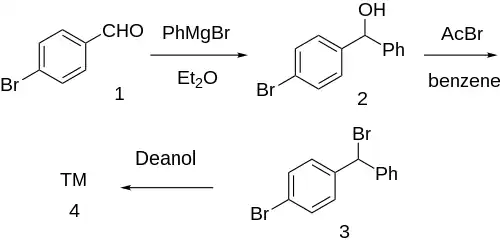
Grignard reaction between phenylmagnesium bromide and para-bromobenzaldehyde [1122-91-4] (1) gives p-bromobenzhydrol [29334-16-5] (2). Halogenation with acetyl bromide in benzene solvent gives p-bromo-benzhydrylbromide [18066-89-2] (3). Finally, etherification with deanol completed the synthesis of Bromazine (4).
Side effects
Continuous and/or cumulative use of anticholinergic medications, including first-generation antihistamines, is associated with higher risk for cognitive decline and dementia in elderly people.[8][9]
References
- J. Elks, ed. (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 177–. ISBN 978-1-4757-2085-3. OCLC 1058412474.
- Swiss Pharmaceutical Society (2000). Swiss Pharmaceutical Society (ed.). Index Nominum 2000: International Drug Directory. Taylor & Francis. pp. 134–. ISBN 978-3-88763-075-1.
- Baker CE (1974). Physicians' Desk Reference (28 ed.). Oradell, NJ 07649: Medical Economics Company. pp. 1076, 1081.
{{cite book}}: CS1 maint: location (link) - Kalpaklioglu F, Baccioglu A (2012). "Efficacy and safety of H1-antihistamines: an update". Anti-Inflamm Anti-Allergy Agents Med Chem. 11 (3): 230–7. doi:10.2174/1871523011202030230. PMID 23173575.
- Maclaren WR, Bruff WC, Eisenberg BC, Weiner H, Martin WH (1955). "A clinical comparison of carbinoxamine maleate, tripelennamine hydrochloride, and bromodiphenhydramine hydrochloride in treating allergic symptoms". Annals of Allergy. 13 (3): 307–12. PMID 14377226.
- Ahmadi, Abbas; Khalili, Mohsen; Hajikhani, Ramin; Safari, Narjes; Nahri-Niknafs, Babak (2011). "Anti-inflammatory effects of two new methyl and morpholine derivatives of diphenhydramine on rats". Medicinal Chemistry Research. 21 (11): 3532–3540. doi:10.1007/s00044-011-9891-y.
- Jr George Rieveschl, US2527963 (1950 to Parke Davis & Co).
- Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. (March 2015). "Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study". JAMA Internal Medicine. 175 (3): 401–407. doi:10.1001/jamainternmed.2014.7663. PMC 4358759. PMID 25621434.
- Carrière, I; Fourrier-Reglat, A; Dartigues, J-F; Rouaud, O; Pasquier, F; Ritchie, K; Ancelin, M-L (July 2009). "Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study". Archives of Internal Medicine. 169 (14): 1317–1324. doi:10.1001/archinternmed.2009.229. PMC 2933398. PMID 19636034.