Bismuth oxychloride
Bismuth oxychloride is an inorganic compound of bismuth with the formula BiOCl. It is a lustrous white solid used since antiquity, notably in ancient Egypt. Light wave interference from its plate-like structure gives a pearly iridescent light reflectivity similar to nacre. It is also known as pearl white.
 | |
| Names | |
|---|---|
| Other names
bismuthyl chloride bismuth oxochloride bismuth oxide chloride bismuth(III) oxide chloride bismoclite | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.202 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| BiOCl | |
| Molar mass | 260.43 g·mol−1 |
| Appearance | Lustrous white crystals with a pearly iridescent light reflectivity |
| Density |
|
| negligible | |
| Solubility | soluble in acids |
| Structure | |
| Tetragonal, tP6[2] | |
| P4/nmm, No. 129 | |
a = 0.3887 nm, c = 0.7354 nm | |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Structure
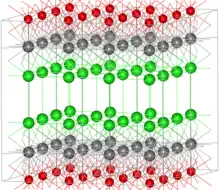
The structure of bismuth oxychloride can be thought of as consisting of layers of Cl−, Bi3+ and O2− ions (in the image Bi = grey, O = red, Cl = green). These ions are ordered as Cl–Bi–O–Bi–Cl–Cl–Bi–O–Bi–Cl, i.e., with alternating anions (Cl−, O2−) and cations (Bi3+). The layered structure gives rise to the pearlescent properties of this material.
Focusing on the coordination environment of the individual ions, the bismuth centers adopt a distorted square antiprismatic coordination geometry. The Bi atom is coordinated to four Cl atoms, forming one of the square faces, each at a distance of 3.06 Å from Bi, and four O atoms forming the other square face, each at a distance of 2.32 Å from Bi. The O atoms are tetrahedrally coordinated by four Bi atoms.[2]
Synthesis and reactions
BiOCl is formed during the reaction of bismuth chloride with water, i.e. the hydrolysis:
- BiCl3 + H2O → BiOCl + 2 HCl
When heated above 600 °C, BiOCl converts to Bi24O31Cl10, called the "Arppe compound" which has a complex layer structure.[3][4]
Use and occurrence
It has been used in cosmetics since the days of ancient Egypt. It is part of the "pearly pigment found in eye shadow, hair sprays, powders, nail polishes, and other cosmetic products".[5] Owing to the plate-like structure of the BiOCl, its suspensions exhibit optical properties like nacre. In cosmetic its name is C.I. 77163.[6]
BiOCl exists in nature as the rare mineral bismoclite, which is part of the matlockite mineral group.[7]
An analogous compound, bismuth oxynitrate, is used as a white pigment.
References
- Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C. (eds.). "Bismoclite". Handbook of Mineralogy (PDF). Vol. III (Halides, Hydroxides, Oxides). Chantilly, VA, US: Mineralogical Society of America. ISBN 0-9622097-2-4. Retrieved December 5, 2011.
- Keramidas, K. G.; Voutsas, G. P.; Rentzeperis, P. I. (1993). "The crystal structure of BiOCl". Zeitschrift für Kristallographie. 205 (Part 1): 35–40. Bibcode:1993ZK....205...35K. doi:10.1524/zkri.1993.205.Part-1.35. ISSN 0044-2968.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 572. ISBN 978-0-08-037941-8.
- Eggenweiler, U.; Keller, E.; Krämer, V. (2000). "Redetermination of the crystal structures of the 'Arppe compound' Bi24O31Cl10 and the isomorphous Bi24O31Br10". Acta Crystallographica Section B. 56 (3): 431–437. doi:10.1107/S0108768100000550. ISSN 0108-7681. PMID 10877351.
- Völz, Hans G. et al. "Pigments, Inorganic" in Ullmann's Encyclopedia of Industrial Chemistry, 2006 Wiley-VCH, Weinheim. doi:10.1002/14356007.a20_243.pub2.
- Carrasco, F. 2009. Diccionario de Ingredientes Cosmeticos(Paperback)
- Bismoclite on Mindat.org