Biphenyl-2,3-diol 1,2-dioxygenase
Biphenyl-2,3-diol 1,2-dioxygenase (EC 1.13.11.39) is an enzyme that catalyzes the chemical reaction
- biphenyl-2,3-diol + O2 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate + H2O
| biphenyl-2,3-diol 1,2-dioxygenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
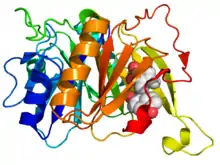 Crystallographic structure of the HsaC extradiol dioxygenase from M. tuberculosis (rainbow colored, N-terminus = blue, C-terminus = red) complexed with 3,4-DHSA (space-filling model (carbon = white, oxygen = red).[1] | |||||||||
| Identifiers | |||||||||
| EC no. | 1.13.11.39 | ||||||||
| CAS no. | 103679-58-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Thus, the two substrates of this enzyme are biphenyl-2,3-diol and oxygen, whereas its two products are 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate and water.
This enzyme belongs to the family of oxidoreductases, specifically those acting on single donors with O2 as oxidant and incorporation of two atoms of oxygen into the substrate (oxygenases). The oxygen incorporated need not be derived from O2. The systematic name of this enzyme class is biphenyl-2,3-diol:oxygen 1,2-oxidoreductase (decyclizing). Other names in common use include 2,3-dihydroxybiphenyl dioxygenase, and biphenyl-2,3-diol dioxygenase. This enzyme participates in gamma-hexachlorocyclohexane degradation and biphenyl degradation.
Structural studies
As of late 2007, 16 structures have been solved for this class of enzymes, with PDB accession codes 1DHY, 1EIL, 1EIQ, 1EIR, 1HAN, 1KMY, 1KND, 1KNF, 1KW3, 1KW6, 1KW8, 1KW9, 1KWB, 1KWC, 1LGT, and 1LKD.
References
- PDB: 2ZI8; Yam KC, D'Angelo I, Kalscheuer R, Zhu H, Wang JX, Snieckus V, Ly LH, Converse PJ, Jacobs WR, Strynadka N, Eltis LD (March 2009). Ramakrishnan L (ed.). "Studies of a Ring-Cleaving Dioxygenase Illuminate the Role of Cholesterol Metabolism in the Pathogenesis of Mycobacterium tuberculosis". PLOS Pathog. 5 (3): e1000344. doi:10.1371/journal.ppat.1000344. PMC 2652662. PMID 19300498.
- Utsumi T; Funatsu G (1986). "Induction of superoxide production in polymorphonuclear leukocytes by ricinus lectins and its dependency on lectin valency". Agric. Biol. Chem. 50 (4): 1047–1049. doi:10.1271/bbb1961.50.1047.