Bathocuproine
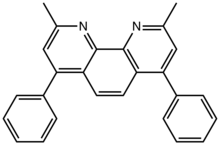
Bathocuproine is a derivative of 1,10-phenanthroline with two methyl groups and two phenyl groups in the 2,9 and 4,7 positions, respectively. Like 1,10-phenanthroline, bathocuproine is a bidentate chelating ligand. The two methyl groups flank the nitrogen centers, such that bathocuproine is a bulky ligand. This compound, first prepared by Case and Brennan in the early 1950s [1][2] is a pale yellow solid that is soluble in polar organic solvents.[3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,9-Dimethyl-4,7-diphenyl-1,10-phenanthroline | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.022.945 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C26H20N2 | |
| Molar mass | 360.460 g·mol−1 |
| Appearance | Pale yellow solid |
| Melting point | 283 °C (541 °F; 556 K) |
| organic solvents | |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H413 | |
| P264, P270, P273, P301+P312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Case, Francis H.; Brennan, James A. (June 1954). "SUBSTITUTED 1, 10-PHENANTHROLINES. VII. SYNTHESIS OF CERTAIN PHENANTHROLINES FOR USE IN THE DETECTION OF Cu(I) 1". The Journal of Organic Chemistry. 19 (6): 919–922. doi:10.1021/jo01371a007.
- Diehl, Harvey; Smith, George Frederick (1972). The Copper Reagents: Cuproine, Neocuproine, Bathocuproine (PDF) (2nd ed.). Columbus, Ohio: G. Frederick Smith Chemical Company. p. 26. Retrieved 1 January 2023.
- Guosheng Liu, Yichen, Wu (2012). "Bathocuproine". Encyclopedia of Reagents for Organic Synthesis. eEROS. doi:10.1002/047084289X.rn01392. ISBN 978-0471936237.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.