Anterior cruciate ligament
The anterior cruciate ligament (ACL) is one of a pair of cruciate ligaments (the other being the posterior cruciate ligament) in the human knee. The two ligaments are also called "cruciform" ligaments, as they are arranged in a crossed formation. In the quadruped stifle joint (analogous to the knee), based on its anatomical position, it is also referred to as the cranial cruciate ligament.[1] The term cruciate translates to cross. This name is fitting because the ACL crosses the posterior cruciate ligament to form an "X". It is composed of strong, fibrous material and assists in controlling excessive motion. This is done by limiting mobility of the joint. The anterior cruciate ligament is one of the four main ligaments of the knee, providing 85% of the restraining force to anterior tibial displacement at 30 and 90° of knee flexion.[2] The ACL is the most injured ligament of the four located in the knee.
| Anterior cruciate ligament | |
|---|---|
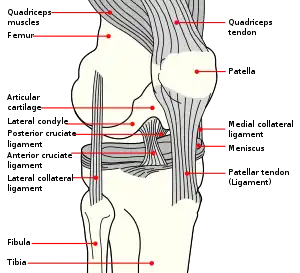 Diagram of = | |
| Details | |
| From | lateral condyle of the femur |
| To | intercondyloid eminence of the tibia |
| Identifiers | |
| Latin | ligamentum cruciatum anterius |
| MeSH | D016118 |
| TA98 | A03.6.08.007 |
| TA2 | 1890 |
| FMA | 44614 |
| Anatomical terminology | |
Structure
The ACL originates from deep within the notch of the distal femur. Its proximal fibers fan out along the medial wall of the lateral femoral condyle.[3] The two bundles of the ACL are the anteromedial and the posterolateral, named according to where the bundles insert into the tibial plateau.[4][5] The tibial plateau is a critical weight-bearing region on the upper extremity of the tibia. The ACL attaches in front of the intercondyloid eminence of the tibia, where it blends with the anterior horn of the medial meniscus.
Purpose
The purpose of the ACL is to resist the motions of anterior tibial translation and internal tibial rotation; this is important to have rotational stability.[6] This function prevents anterior tibial subluxation of the lateral and medial tibiofemoral joints, which is important for the pivot-shift phenomenon.[6] The ACL has mechanoreceptors that detect changes in direction of movement, position of the knee joint, and changes in acceleration, speed, and tension.[7] A key factor in instability after ACL injuries is having altered neuromuscular function secondary to diminished somatosensory information.[7] For athletes who participate in sports involving cutting, jumping, and rapid deceleration, the knee must be stable in terminal extension, which is the screw-home mechanism.[7]
Clinical significance
Injury
An ACL tear is one of the most common knee injuries, with over 100,000 tears occurring annually in the US.[8] Most ACL tears are a result of a non-contact mechanism such as a sudden change in a direction causing the knee to rotate inward. As the knee rotates inward, additional strain is placed on the ACL, since the femur and tibia, which are the two bones that articulate together forming the knee joint, move in opposite directions, causing the ACL to tear. Most athletes require reconstructive surgery on the ACL, in which the torn or ruptured ACL is completely removed and replaced with a piece of tendon or ligament tissue from the patient (autograft) or from a donor (allograft).[9] Conservative treatment has poor outcomes in ACL injury, since the ACL is unable to form a fibrous clot, as it receives most of its nutrients from synovial fluid; this washes away the reparative cells, making the formation of fibrous tissue difficult. The two most common sources for tissue are the patellar ligament and the hamstrings tendon.[10] The patellar ligament is often used, since bone plugs on each end of the graft are extracted, which helps integrate the graft into the bone tunnels during reconstruction.[11] The surgery is arthroscopic, meaning that a tiny camera is inserted through a small surgical cut.[9] The camera sends video to a large monitor so the surgeon can see any damage to the ligaments. In the event of an autograft, the surgeon makes a larger cut to get the needed tissue. In the event of an allograft, in which material is donated, this is not necessary, since no tissue is taken directly from the patient's own body.[12] The surgeon drills a hole forming the tibial bone tunnel and femoral bone tunnel, allowing for the patient's new ACL graft to be guided through.[12] Once the graft is pulled through the bone tunnels, two screws are placed into the tibial and femoral bone tunnel.[12] Recovery time usually ranges between one and two years, but is sometimes longer, depending if the patient chose an autograft or allograft. A week or so after the occurrence of the injury, the athlete is usually deceived by the fact that he/she is walking normally and not feeling much pain.[12] This is dangerous, as some athletes start resuming some of their activities such as jogging, which with a wrong move or twist, could damage the bones, as the graft has not completely become integrated into the bone tunnels. Injured athletes must understand the significance of each step of an ACL injury to avoid complications and ensure a proper recovery.
Nonoperative treatment of the ACL
ACL reconstruction is the most common treatment for an ACL tear, but it is not the only treatment available for individuals. Some may find it more beneficial to complete a nonoperative rehabilitation program. Individuals who are going to continue with physical activity that involves cutting and pivoting, and individuals who are no longer participating in those specific activities both are candidates for the nonoperative route.[13] In comparing operative and nonoperative approaches to ACL tears, few differences were noted between surgical and nonsurgical groups, with no significant differences in regard to knee function or muscle strength reported by the patients.
The main goals to achieve during rehabilitation (rehab) of an ACL tear is to regain sufficient functional stability, maximize full muscle strength, and decrease risk of reinjury. Typically, three phases are involved in nonoperative treatment - the acute phase, the neuromuscular training phase, and the return to sport phase. During the acute phase, the rehab is focusing on the acute symptoms that occur right after the injury and are causing an impairment. The use of therapeutic exercises and appropriate therapeutic modalities is crucial during this phase to assist in repairing the impairments from the injury. The neuromuscular training phase is used to focus on the patient regaining full strength in both the lower extremity and the core muscles. This phase begins when the patient regains full range of motion, no effusion, and adequate lower extremity strength. During this phase, the patient completes advanced balance, proprioception, cardiovascular conditioning, and neuromuscular interventions.[13] In the final, return to sport phase, the patient focuses on sport-specific activities and agility. A functional performance brace is suggested to be used during the phase to assist with stability during pivoting and cutting activities.[13]
Operative treatment of the ACL
Anterior cruciate ligament surgery is a complex operation that requires expertise in the field of orthopedic and sports medicine. Many factors should be considered when discussing surgery, including the athlete's level of competition, age, previous knee injury, other injuries sustained, leg alignment, and graft choice. Typically, four graft types are possible, the bone-patella tendon-bone graft, the semitendinosus and gracilis tendons (quadrupled hamstring tendon), quadriceps tendon, and an allograft.[14] Although extensive research has been conducted on which grafts are the best, the surgeon typically chooses the type of graft with which he or she is most comfortable. If rehabilitated correctly, the reconstruction should last. In fact, 92.9% of patients are happy with graft choice.[14]
Prehabilitation has become an integral part of the ACL reconstruction process. This means that the patient exercises before getting surgery to maintain factors such as range of motion and strength. Based on a single leg hop test and self-reported assessment, prehab improved function; these effects were sustained 12 weeks postoperatively.[15]
Postsurgical rehabilitation is essential in the recovery from the reconstruction. This typically takes a patient 6 to 12 months to return to life as it was prior to the injury.[16] The rehab can be divided into protection of the graft, improving range of motion, decrease swelling, and regaining muscle control.[16] Each phase has different exercises based on the patients' needs. For example, while the ligament is healing, a patient's joint should not be used for full weight-bearing, but the patient should strengthen the quadriceps and hamstrings by doing quad sets and weight shifting drills. Phase two would require full weight-bearing and correcting gait patterns, so exercises such as core strengthening and balance exercises would be appropriate. In phase three, the patient begins running, and can do aquatic workouts to help with reducing joint stresses and cardiorespiratory endurance. Phase four includes multiplanar movements, thus enhancing a running program and beginning agility and plyometric drills. Lastly, phase five focuses on sport- or life-specific motions, depending on the patient.[16]
A 2010 Los Angeles Times review of two medical studies discussed whether ACL reconstruction was advisable. One study found that children under 14 who had ACL reconstruction fared better after early surgery than those who underwent a delayed surgery. For adults 18 to 35, though, patients who underwent early surgery followed by rehabilitation fared no better than those who had rehabilitative therapy and a later surgery.[17]
The first report focused on children and the timing of an ACL reconstruction. ACL injuries in children are a challenge because children have open growth plates in the bottom of the femur or thigh bone and on the top of the tibia or shin. An ACL reconstruction typically crosses the growth plates, posing a theoretical risk of injury to the growth plate, stunting leg growth, or causing the leg to grow at an unusual angle.[18]
The second study noted focused on adults. It found no significant statistical difference in performance and pain outcomes for patients who receive early ACL reconstruction vs. those who receive physical therapy with an option for later surgery. This would suggest that many patients without instability, buckling, or giving way after a course of rehabilitation can be managed nonoperatively, but was limited to outcomes after two years and did not involve patients who were serious athletes.[17] Patients involved in sports requiring significant cutting, pivoting, twisting, or rapid acceleration or deceleration may not be able to participate in these activities without ACL reconstruction.[19]
ACL injuries in women
Risk differences between outcomes in men and women can be attributed to a combination of multiple factors, including anatomical, hormonal, genetic, positional, neuromuscular, and environmental factors.[20] The size of the anterior cruciate ligament is often the most reported difference. Studies look at the length, cross-sectional area, and volume of ACLs. Researchers use cadavers, and in vivo placement to study these factors, and most studies confirm that women have smaller anterior cruciate ligaments. Other factors that could contribute to higher risks of ACL tears in women include patient weight and height, the size and depth of the intercondylar notch, the diameter of the ACL, the magnitude of the tibial slope, the volume of the tibial spines, the convexity of the lateral tibiofemoral articular surfaces, and the concavity of the medial tibial plateau.[21] While anatomical factors are most talked about, extrinsic factors, including dynamic movement patterns, might be the most important risk factor when it comes to ACL injury.[22]
Gallery
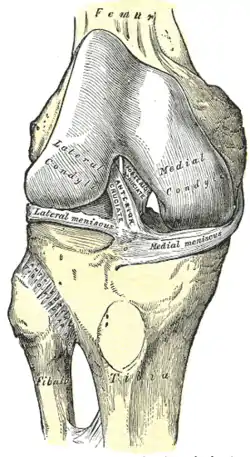 Right knee joint, from the front, showing interior ligaments
Right knee joint, from the front, showing interior ligaments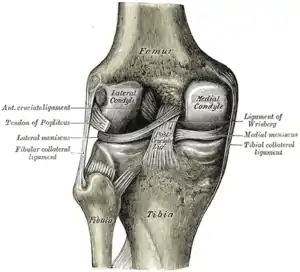 Left knee joint from behind, showing interior ligaments
Left knee joint from behind, showing interior ligaments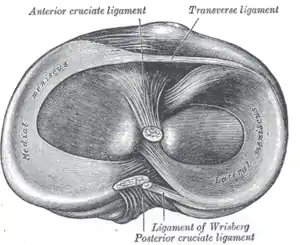 Head of right tibia seen from above, showing menisci and attachments of ligaments
Head of right tibia seen from above, showing menisci and attachments of ligaments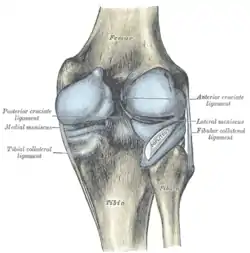 Capsule of right knee-joint (distended), posterior aspect
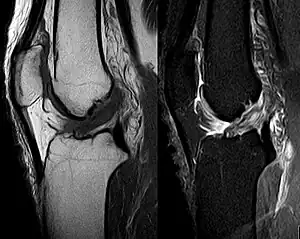
Capsule of right knee-joint (distended), posterior aspect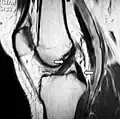 MRI shows normal signal of both cruciate ligaments (arrows)
MRI shows normal signal of both cruciate ligaments (arrows)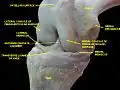 Knee joint, deep dissection, anteromedial view
Knee joint, deep dissection, anteromedial view
See also
References
- "Canine Cranial Cruciate Ligament Disease" (PDF). Melbourne Veterinary Referral Centre. pp. 1–2. Archived from the original (PDF) on 19 July 2008. Retrieved September 8, 2009.
- Ellison, A. E.; Berg, E. E. (1985). "Embryology, anatomy, and function of the anterior cruciate ligament". The Orthopedic Clinics of North America. 16 (1): 3–14. doi:10.1016/S0030-5898(20)30463-6. PMID 3969275.
- Petersen, W.; Tillmann, B. (August 2002). "[Anatomy and function of the anterior cruciate ligament]". Der Orthopade. 31 (8): 710–718. doi:10.1007/s00132-002-0330-0. ISSN 0085-4530. PMID 12426749. S2CID 45919449.
- Duthon, V. B.; Barea, C.; Abrassart, S.; Fasel, J. H.; Fritschy, D.; Ménétrey, J. (March 2006). "Anatomy of the anterior cruciate ligament". Knee Surgery, Sports Traumatology, Arthroscopy. 14 (3): 204–213. doi:10.1007/s00167-005-0679-9. ISSN 0942-2056. PMID 16235056. S2CID 25658911.
- Petersen, Wolf; Zantop, Thore (January 2007). "Anatomy of the anterior cruciate ligament with regard to its two bundles". Clinical Orthopaedics and Related Research. 454: 35–47. doi:10.1097/BLO.0b013e31802b4a59. ISSN 0009-921X. PMID 17075382.
- Noyes, Frank R. (January 2009). "The Function of the Human Anterior Cruciate Ligament and Analysis of Single- and Double-Bundle Graft Reconstructions". Sports Health. 1 (1): 66–75. doi:10.1177/1941738108326980. ISSN 1941-7381. PMC 3445115. PMID 23015856.
- Liu-Ambrose, T. (December 2003). "The anterior cruciate ligament and functional stability of the knee joint". British Columbia Medical Journal. 45 (10): 495–499. Retrieved 2018-11-15.
- Cimino, Francesca; Volk, Bradford Scott; Setter, Don (2010-10-15). "Anterior Cruciate Ligament Injury: Diagnosis, Management, and Prevention". American Family Physician. 82 (8): 917–922. ISSN 0002-838X. PMID 20949884.
- "ACL reconstruction - Mayo Clinic". www.mayoclinic.org. Retrieved 2018-11-15.
- Samuelsen, Brian T.; Webster, Kate E.; Johnson, Nick R.; Hewett, Timothy E.; Krych, Aaron J. (October 2017). "Hamstring Autograft versus Patellar Tendon Autograft for ACL Reconstruction: Is There a Difference in Graft Failure Rate? A Meta-analysis of 47,613 Patients". Clinical Orthopaedics and Related Research. 475 (10): 2459–2468. doi:10.1007/s11999-017-5278-9. ISSN 1528-1132. PMC 5599382. PMID 28205075.
- "When Would You Use Patellar Tendon Autograft as Your Main Graft Selection?". www.healio.com. Retrieved 2018-11-15.
- "ACL Injury: Does It Require Surgery? - OrthoInfo - AAOS". Retrieved 2018-11-15.
- Paterno, Mark V. (2017-07-29). "Non-operative Care of the Patient with an ACL-Deficient Knee". Current Reviews in Musculoskeletal Medicine. 10 (3): 322–327. doi:10.1007/s12178-017-9431-6. ISSN 1935-973X. PMC 5577432. PMID 28756525.
- Macaulay, Alec A.; Perfetti, Dean C.; Levine, William N. (January 2012). "Anterior Cruciate Ligament Graft Choices". Sports Health. 4 (1): 63–68. doi:10.1177/1941738111409890. ISSN 1941-7381. PMC 3435898. PMID 23016071.
- Shaarani, Shahril R.; O'Hare, Christopher; Quinn, Alison; Moyna, Niall; Moran, Raymond; O'Byrne, John M. (September 2013). "Effect of prehabilitation on the outcome of anterior cruciate ligament reconstruction". The American Journal of Sports Medicine. 41 (9): 2117–2127. doi:10.1177/0363546513493594. ISSN 1552-3365. PMID 23845398. S2CID 38240767.
- "Rehabilitation Guidelines for ACL Reconstruction in the Adult Athlete (Skeletally Mature)" (PDF). UW Health. Archived from the original (PDF) on 2020-11-12. Retrieved 2018-12-06.
- Stein, Jeannine (2010-07-22). "Studies on ACL surgery". The Los Angeles Times. Archived from the original on July 28, 2010. Retrieved 2010-07-23.
- "ACL Tears: To reconstruct or not, and if so, when?". howardluksmd.com. Archived from the original on January 25, 2013. Retrieved 2010-07-23.
- Frobell, Richard B.; Roos, Ewa M.; Roos, Harald P.; Ranstam, Jonas; Lohmander, L. Stefan (2010). "A Randomized Trial of Treatment for Acute Anterior Crut Tears". New England Journal of Medicine. 363 (4): 331–342. doi:10.1056/NEJMoa0907797. PMID 20660401.
- Chandrashekara, Naveen; Mansourib, Hossein; Slauterbeckc, James; Hashemia, Javad (2006). "Sex-based differences in the tensile properties of the human anterior cruciate ligament". Journal of Biomechanics. 39 (16): 2943–2950. doi:10.1016/j.jbiomech.2005.10.031. PMID 16387307.
- Schneider, Antione; Si-Mohamed, Salim; Magnussen, Robert; Lustig, Sebastien; Neyret, Philippe; Servien, Elvire (October 2017). "Tibiofemoral joint congruence is lower in females with ACL injuries than males with ACL injuries". Knee Surgery, Sports Traumatology, Arthroscopy. 25 (5): 1375–1383. doi:10.1007/s00167-017-4756-7. PMID 29052744. S2CID 4968334.
- Lloyd Ireland, Mary (2002). "The female ACL: why is it more prone to injury?". Orthopedic Clinics of North America. 33 (2): 637–651. doi:10.1016/S0030-5898(02)00028-7. PMC 4805849. PMID 12528906.
External links
- Anatomy photo:17:02-0701 at the SUNY Downstate Medical Center - "Extremity: Knee joint"
- Anatomy figure: 17:07-08 at Human Anatomy Online, SUNY Downstate Medical Center - "Superior view of the tibia."
- Anatomy figure: 17:08-03 at Human Anatomy Online, SUNY Downstate Medical Center - "Medial and lateral views of the knee joint and cruciate ligaments."
- lljoints at The Anatomy Lesson by Wesley Norman (Georgetown University) (antkneejointopenflexed)