Amidase
In enzymology, an amidase (EC 3.5.1.4, acylamidase, acylase (misleading), amidohydrolase (ambiguous), deaminase (ambiguous), fatty acylamidase, N-acetylaminohydrolase (ambiguous)) is an enzyme that catalyzes the hydrolysis of an amide. In this way, the two substrates of this enzyme are an amide and H2O, whereas its two products are monocarboxylate and NH3.
| Amidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 X-ray structure of native peptide amidase from Stenotrophomonas maltophilia at 1.4 Å | |||||||||
| Identifiers | |||||||||
| Symbol | Amidase | ||||||||
| Pfam | PF01425 | ||||||||
| InterPro | IPR000120 | ||||||||
| PROSITE | PDOC00494 | ||||||||
| SCOP2 | 1ocm / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 55 | ||||||||
| OPM protein | 1mt5 | ||||||||
| Membranome | 325 | ||||||||
| |||||||||
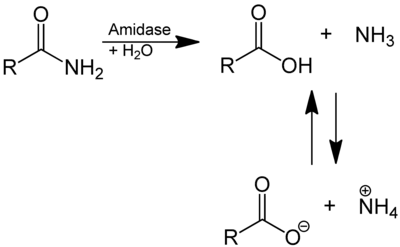
This enzyme belongs to the family of hydrolases, those acting on carbon-nitrogen bonds other than peptide bonds, specifically in linear amides. The systematic name of this enzyme class is acylamide amidohydrolase. Other names in common use include acylamidase, acylase, amidohydrolase, deaminase, fatty acylamidase, and N-acetylaminohydrolase. This enzyme participates in 6 metabolic pathways: urea cycle and metabolism of amino groups, phenylalanine metabolism, tryptophan metabolism, cyanoamino acid metabolism, benzoate degradation via coa ligation, and styrene degradation.
Amidases contain a conserved stretch of approximately 130 amino acids known as the AS sequence. They are widespread, being found in both prokaryotes and eukaryotes. AS enzymes catalyse the hydrolysis of amide bonds (CO-NH2), although the family has diverged widely with regard to substrate specificity and function. Nonetheless, these enzymes maintain a core alpha/beta/alpha structure, where the topologies of the N- and C-terminal halves are similar. AS enzymes characteristically have a highly conserved C-terminal region rich in serine and glycine residues, but devoid of aspartic acid and histidine residues, therefore they differ from classical serine hydrolases. These enzymes possess a unique, highly conserved Ser-Ser-Lys catalytic triad used for amide hydrolysis, although the catalytic mechanism for acyl-enzyme intermediate formation can differ between enzymes.[1]
Examples of AS signature-containing enzymes include:
- Peptide amidase (Pam),[1] which catalyses the hydrolysis of the C-terminal amide bond of peptides.
- Fatty acid amide hydrolases,[2] which hydrolyse fatty acid amid substrates (e.g. cannabinoid anandamide and sleep-inducing oleamide), thereby controlling the level and duration of signalling induced by this diverse class of lipid transmitters.
- Malonamidase E2,[3] which catalyses the hydrolysis of malonamate into malonate and ammonia, and which is involved in the transport of fixed nitrogen from bacteroids to plant cells in symbiotic nitrogen metabolism.
- Subunit A of Glu-tRNA(Gln) amidotransferase,[4] a heterotrimeric enzyme that catalyses the formation of Gln-tRNA(Gln) by the transamidation of misacylated Glu-tRNA(Gln) via amidolysis of glutamine.
Structural studies
As of late 2018, 162 structures have been solved for this family, which can be accessed at the Pfam Archived 2021-09-18 at the Wayback Machine.
References
- Valiña AL, Mazumder-Shivakumar D, Bruice TC (December 2004). "Probing the Ser-Ser-Lys catalytic triad mechanism of peptide amidase: computational studies of the ground state, transition state, and intermediate". Biochemistry. 43 (50): 15657–72. doi:10.1021/bi049025r. PMID 15595822.
- Wei BQ, Mikkelsen TS, McKinney MK, Lander ES, Cravatt BF (December 2006). "A second fatty acid amide hydrolase with variable distribution among placental mammals". J. Biol. Chem. 281 (48): 36569–78. doi:10.1074/jbc.M606646200. PMID 17015445.
- Shin S, Lee TH, Ha NC, Koo HM, Kim SY, Lee HS, Kim YS, Oh BH (June 2002). "Structure of malonamidase E2 reveals a novelSer-cisSer-Lys catalytic triad in a new serine hydrolase fold that is prevalent in nature". EMBO J. 21 (11): 2509–16. doi:10.1093/emboj/21.11.2509. PMC 126024. PMID 12032064.
- Kwak JH, Shin K, Woo JS, Kim MK, Kim SI, Eom SH, Hong KW (December 2002). "Expression, purification, and crystallization of glutamyl-tRNA(Gln) specific amidotransferase from Bacillus stearothermophilus". Mol. Cells. 14 (3): 374–81. PMID 12521300.
Further reading
- Bray HG, James SP, Raffan IM, Ryman BE, Thorpe WV (1949). "The fate of certain organic acids and amides in the rabbit. 7. An amidase of rabbit liver". Biochem. J. 44 (5): 618–625. doi:10.1042/bj0440618. PMC 1274617.
- Bray HG, James SP, Thorpe WV, Wasdell MR (1950). "The fate of certain organic acids and amides in the rabbit. 11 Further observations on the hydrolysis of amides by tissue extracts". Biochem. J. 47 (3): 294–299. doi:10.1042/bj0470294. PMC 1275209. PMID 14800883.