α-Isomethyl ionone
α-Isomethyl ionone, also known as α-cetone, is a synthetically made and naturally occurring organic compound found in Brewer's yeasts or the species known as Saccharomyces cerevisiae.[1] The compound is an isomer of methyl ionone. Alpha-isomethyl ionone can be colorless or pale-straw coloured liquid.[2] Its primary scent is flowery and secondary scent is violet. It may also have a woody or orris-like scent.[3] and is often used in flavouring and cosmetic industries for example, aftershave lotions, bath products, hair care products, moisturizers, perfumes, shampoos and skin care products.[4] It is also an ingredient used in Chanel No. 5,[5] and other branded products such as Fidji by Guy Laroche.[6] Perfume fragrances that α-isomethyl ionone is used in are for example, amber, chypre, violet, mimosa, reseda, iris, orris, cyclamen, chypre, berries, woody notes, ylang-ylang, leather, orange, nut, pistachio, muscatel, and tobacco.[6]
 | |
 | |
| Names | |
|---|---|
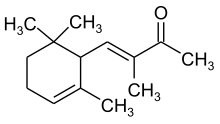
| IUPAC name
(3E)-3-methyl-4-(2,6,6-trimethylcyclohex-2-en-1-yl)but-3-en-2-one | |
| Other names
α-Cetone Cetone Alpha Isomethyl-α-ionone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.407 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H22O | |
| Molar mass | 206.3239 |
| Appearance | liquid |
| Density | 0.93 gcm −3 (20 °C) |
| Boiling point | 93 °C (199 °F; 366 K) (3.1 mmHg) |
| 0.064 g/L | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
irritant, environmental hazard |
| GHS labelling: | |
 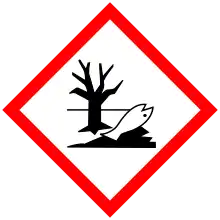 | |
| Warning | |
| H315, H317, H319, H411, H412 | |
| P261, P264, P272, P273, P280, P302+P352, P305+P351+P338, P321, P332+P313, P333+P313, P337+P313, P362, P363, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Properties

α-Isomethyl ionone would be classified as a norsesquiterpenoid, having 14 carbon atoms (1 less than the 15 of three consecutive isoprene units). It is an extremely weak base, the calculated pKa values within the molecule being 19.7 (strongest acidic) and -4.8 (strongest basic).[7] The percentage of α-isomethyl ionone used in perfumes is approximately ranging from 0.1% to 11.9%, with an average of 1.1%. For example, it is usually used in conjunction with hydroxycitronellal, woody notes, copaiba, N-methyl ionone, ionone, or Vetiver.[6]
Synthesis
The synthesis of α-isomethyl ionone involves a cross-aldol condensation of citral with methyl ethyl ketone[8] A high temperature and strong alkali is used. The ratio between the n-form and iso-form is controlled in order to obtain methyl pseudo-ionone and allow ring formation to occur. Iso-forms is then synthesized consequently.[9]
References
- "alpha-Isomethyl-ionone (YMDB01634)". YMDB. Retrieved 29 May 2020.
- "Alpha-Isomethyl Ionone". Cosmetic Info. Retrieved 29 May 2020.
- "Frangrance Demo Formula". TGSC Information System. Retrieved 24 June 2020.
- "Alfa-Isomethyl ionone". Chemo Technique Diagnostics. Retrieved 29 May 2020.
- Kennedy, James. "Visual Ingredients Chanel No. 5" (PDF). James Kennedy Monash. Retrieved 29 May 2020.
- "Alpha Isomethyl Ionone". Perfumes World. Retrieved 9 July 2020.
- "3-Methyl-4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-one". Foodb. Retrieved 9 July 2020.
- Burdock, George A. (29 July 2014). Encyclopedia of Food and Colour additives. ISBN 9781498711081. Retrieved 13 July 2020.
- "ALPHA-ISO-METHYLIONONE". Chemical Book. Retrieved 24 June 2020.