Albumin
Albumin is a family of globular proteins, the most common of which are the serum albumins. All of the proteins of the albumin family are water-soluble, moderately soluble in concentrated salt solutions, and experience heat denaturation. Albumins are commonly found in blood plasma and differ from other blood proteins in that they are not glycosylated. Substances containing albumins are called albuminoids.
| Serum albumin family | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||
| Identifiers | |||||||||||
| Symbol | Serum_albumin | ||||||||||
| Pfam | PF00273 | ||||||||||
| Pfam clan | CL0282 | ||||||||||
| InterPro | IPR014760 | ||||||||||
| SMART | SM00103 | ||||||||||
| PROSITE | PS51438 | ||||||||||
| SCOP2 | 1ao6 / SCOPe / SUPFAM | ||||||||||
| |||||||||||
A number of blood transport proteins are evolutionarily related in the albumin family, including serum albumin, alpha-fetoprotein, vitamin D-binding protein and afamin.[3][4][5] This family is only found in vertebrates.[6]
Albumins in a less strict sense can mean other proteins that coagulate under certain conditions. See § Other albumin types for lactalbumin, ovalbumin and plant "2S albumin".
Function
Albumins in general are transport proteins that bind to various ligands and carry them around.[6] Human types include:
- Human serum albumin is the main protein of human blood plasma. It makes up around 50% of human plasma proteins. It binds water, cations (such as Ca2+, Na+ and K+), fatty acids, hormones, bilirubin, thyroxine (T4) and pharmaceuticals (including barbiturates). Its main function is to regulate the oncotic pressure of blood.[7] The isoelectric point of albumin is 4.7.[8]
- Alpha-fetoprotein is a fetal plasma protein that binds various cations, fatty acids and bilirubin.
- Vitamin D-binding protein binds to vitamin D and its metabolites, as well as to fatty acids.
- Not much is known about afamin. It seems to carry lipidated Wnt proteins and Vitamin E around.[9]
- Extracellular matrix protein 1 is a less canonical albumin. It regulates bone mineralization.
The four canonical human albumins are arranged on chromosome 4 region 4q13.3 in a tandem manner.[10]
Classification
Albumins found in animals can be divided into six subfamilies by phylogeny. The Vitamin-D binding proteins occupy families 1–3. The other albumins are mixed among each other in families 4–6. ECM1 is in family 6.[6]
In addition to their medical use, serum albumins are valued in biotechnology. Bovine serum albumin is usually used, although versions from humans and genetically-modified rice are also used to reduce animal cruelty.
Other albumin types
A few other proteins are also sometimes called albumins. They are not in the same family as vertebrate albumins:
- Ovalbumin is a storage protein in egg white (albumen). It is a serpin.
- Lactalbumin, or whey protein, is a protein fraction of milk. It is mainly Beta-lactoglobulin, although serum albumin also comprises a small part of it.
- Some plant seeds, including hemp, encode "2S albumins". These are named for their egg-like coagulation property.[11]
Structure
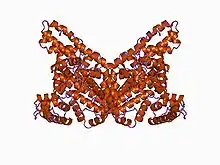
The 3D structure of human serum albumin has been determined by X-ray crystallography to a resolution of 2.5 ångströms (250 pm).[1] Albumin is a 65–70 kDa protein.
Albumin comprises three homologous domains that assemble to form a heart-shaped protein.[2] Each domain is a product of two subdomains that possess common structural motifs.[2] The principal regions of ligand binding to human serum albumin are located in hydrophobic cavities in subdomains IIA and IIIA, which exhibit similar chemistry. Structurally, the serum albumins are similar, each domain containing five or six internal disulfide bonds.
Forensic uses
Worldwide, certain traditional Chinese medicines contain wild bear bile, banned under CITES legislation. Dip sticks, similar to common pregnancy tests, have been developed to detect the presence of bear albumin in traditional medicine products, indicating that bear bile had been used in their creation.[12]
Terminology
Albumin is pronounced /ˈælbjʊmɪn/; formed from Latin: albumen[13] "(egg) white; dried egg white".
See also
- Cohn process (human serum albumin purification method)
- Serum albumin
References
- Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K (June 1999). "Crystal structure of human serum albumin at 2.5 A resolution". Protein Engineering. 12 (6): 439–46. doi:10.1093/protein/12.6.439. PMID 10388840.
- He XM, Carter DC (July 1992). "Atomic structure and chemistry of human serum albumin". Nature. 358 (6383): 209–15. Bibcode:1992Natur.358..209H. doi:10.1038/358209a0. PMID 1630489. S2CID 4353741.
- Haefliger DN, Moskaitis JE, Schoenberg DR, Wahli W (October 1989). "Amphibian albumins as members of the albumin, alpha-fetoprotein, vitamin D-binding protein multigene family". Journal of Molecular Evolution. 29 (4): 344–54. Bibcode:1989JMolE..29..344H. doi:10.1007/BF02103621. PMID 2481749. S2CID 1456034.
- Schoentgen F, Metz-Boutigue MH, Jollès J, Constans J, Jollès P (June 1986). "Complete amino acid sequence of human vitamin D-binding protein (group-specific component): evidence of a three-fold internal homology as in serum albumin and alpha-fetoprotein". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 871 (2): 189–98. doi:10.1016/0167-4838(86)90173-1. PMID 2423133.
- Lichenstein HS, Lyons DE, Wurfel MM, Johnson DA, McGinley MD, Leidli JC, et al. (July 1994). "Afamin is a new member of the albumin, alpha-fetoprotein, and vitamin D-binding protein gene family". The Journal of Biological Chemistry. 269 (27): 18149–54. doi:10.1016/S0021-9258(17)32429-8. PMID 7517938.
- Li S, Cao Y, Geng F (2017). "Genome-Wide Identification and Comparative Analysis of Albumin Family in Vertebrates". Evolutionary Bioinformatics Online. 13: 1176934317716089. doi:10.1177/1176934317716089. PMC 5480655. PMID 28680266.
- Farrugia A (January 2010). "Albumin usage in clinical medicine: tradition or therapeutic?". Transfusion Medicine Reviews. 24 (1): 53–63. doi:10.1016/j.tmrv.2009.09.005. PMID 19962575.
- "Product Information data sheet" (PDF). Sigma Aldrich.
- Mihara E, Hirai H, Yamamoto H, Tamura-Kawakami K, Matano M, Kikuchi A, et al. (February 2016). "Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/α-albumin". eLife. 5. doi:10.7554/eLife.11621. PMC 4775226. PMID 26902720.
- Nishio H, Heiskanen M, Palotie A, Bélanger L, Dugaiczyk A (May 1996). "Tandem arrangement of the human serum albumin multigene family in the sub-centromeric region of 4q: evolution and chromosomal direction of transcription". Journal of Molecular Biology. 259 (1): 113–9. doi:10.1006/jmbi.1996.0306. PMID 8648639.
- Shewry PR, Pandya MJ (1999). "The 2S Albumin Storage Proteins". Seed Proteins. Springer Netherlands. pp. 563–586. doi:10.1007/978-94-011-4431-5_24. ISBN 978-94-011-4431-5.
- Peppin L, McEwing R, Webster S, Rogers A, Nicholls D, Ogden R (September 2008). "Development of a field test for the detection of illegal bear products" (PDF). Endangered Species Research. 9 (3): 263–70. doi:10.3354/esr00131.
- Bostock J. "Pliny the Elder, The Natural History". Historia Naturalis 28, 6, 18.
External links
- Albumins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- The Albumin website
- Albumin binding prediction
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Serum albumin.