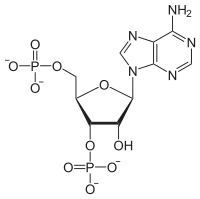
Adenosine 3',5'-bisphosphate
Adenosine 3',5'-bisphosphate is a form of an adenosine nucleotide with two phosphate groups attached to different carbons in the ribose ring. This is distinct from adenosine diphosphate, where the two phosphate groups are attached in a chain to the 5' carbon atom in the ring.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Adenosine 3′,5′-bis(dihydrogen phosphate) | |
| Systematic IUPAC name
(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-2-[(phosphonooxy)methyl]oxolan-3-yl dihydrogen phosphate | |
| Other names
3'-Phosphoadenylate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| MeSH | Adenosine+bisphosphate |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H15N5O10P2 | |
| Molar mass | 427.20 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Adenosine 3',5'-bisphosphate is produced as a product of sulfotransferase enzymes from the donation of a sulfate group from the coenzyme 3'-phosphoadenosine-5'-phosphosulfate.[1][2] This product is then hydrolysed by 3'(2'),5'-bisphosphate nucleotidase to give adenosine monophosphate, which can then be recycled into adenosine triphosphate.[3][4]
See also
References
- Negishi M, Pedersen LG, Petrotchenko E, et al. (2001). "Structure and function of sulfotransferases". Arch. Biochem. Biophys. 390 (2): 149–57. doi:10.1006/abbi.2001.2368. PMID 11396917.
- Rath VL, Verdugo D, Hemmerich S (2004). "Sulfotransferase structural biology and inhibitor discovery". Drug Discov. Today. 9 (23): 1003–11. doi:10.1016/S1359-6446(04)03273-8. PMID 15574316.
- Farooqui AA, Balasubramanian AS (1970). "Enzymatic dephosphorylation 3'-phosphoadenosine 5'-phoaphosulfate to adenosine 5'-phosphosulfate in sheep brain". Biochim. Biophys. Acta. 198 (1): 56–65. doi:10.1016/0005-2744(70)90032-x. PMID 4313079.
- Ramaswamy SG, Jakoby WB (1987). "(2')3',5'-Bisphosphate nucleotidase". J. Biol. Chem. 262 (21): 10044–7. doi:10.1016/S0021-9258(18)61072-5. PMID 3038862.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.