4-Vinylbenzyl chloride
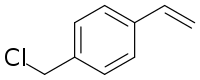
4-Vinylbenzyl chloride is an organic compound with the formula ClCH2C6H4CH=CH2. It is a bifunctional molecule, featuring both vinyl and a benzylic chloride functional groups. It is a colorless liquid that is typically stored with a stabilizer to suppress polymerization.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-(Chloromethyl)-4-ethenylbenzene | |
| Other names
1-(Chloromethyl)-4-vinylbenzene α-Chloromethylstyrene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.014.975 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H9Cl | |
| Molar mass | 152.62 |
| Appearance | colorless liquid |
| Density | 1.083 |
| Boiling point | 229 °C (444 °F; 502 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
alkylating agent |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In combination with styrene, vinylbenzyl chloride is used as a comonomer in the production of chloromethylated polystyrene.[1] It is produced by the chlorination of vinyltoluene. Often vinyltoluene consists of a mixture of 3- and 4-vinyl isomers, in which case the vinylbenzyl chloride will also be produced as a mixture of isomers.[2]
References
- Montheard, Jean Pierre; Jegat, Corinne; Camps, Marcel "Vinylbenzylchloride (chloromethylstyrene), polymers, and copolymers. Recent reactions and applications" Journal of Macromolecular Science, Reviews in Macromolecular Chemistry and Physics 1999, volume C39, pp. 135-174.
- Denis H. James; William M. Castor (2007), "Styrene", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 1, doi:10.1002/14356007.a25_329.pub2, ISBN 978-3527306732
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.