2,3-Epoxybutane
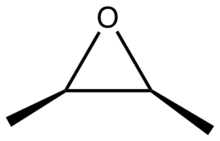
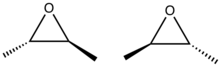
2,3-Epoxybutane is an organic compound with the formula CH3CH(O)CHCH3. It is an epoxide. The compound exists as three stereoisomers, a pair of enantiomers and the meso isomer. All are colorless liquids.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dimethyloxirane | |
| Other names
2,3-Butyleneoxide, 2,3-Buteneoxide | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ECHA InfoCard | 100.019.889 |
| EC Number |
|
PubChem CID |
|
| UN number | 3271 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H8O | |
| Molar mass | 72.107 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.837 g·cm−3 |
| Boiling point | 64–78 °C (147–172 °F; 337–351 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation and reactions
2,3-Epoxybutane is prepared from 2-butene via the chlorohydrin:[1]
- CH3CH=CHCH3 + HOCl → CH3CH(OH)CH(Cl)CH3
- CH3CH(OH)CH(Cl)CH3 → CH3CH(O)CHCH3 + HCl
A common reaction is its hydration to 2,3-butanediol. Many such ring-opening reactions have been reported.[2]
References
- Heinz Gräfje, Wolfgang Körnig, Hans-Martin Weitz, Wolfgang Reiß, Guido Steffan, Herbert Diehl, Horst Bosche, Kurt Schneider and Heinz Kieczka "Butanediols, Butenediol, and Butynediol" in Ullmann's Encyclopedia of Industrial Chemistry, 2019, Wiley-VCH, Weinheim. doi:10.1002/14356007.a04_455.pub2
- Nugent, William A. (1998). "Desymmetrization of Meso Epoxides with Halides: A New Catalytic Reaction Based on Mechanistic Insight". Journal of the American Chemical Society. 120 (28): 7139–7140. doi:10.1021/JA981031L.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.