2,3,4-Pentanetrione
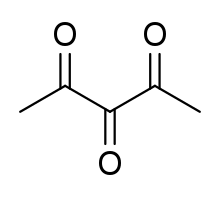
2,3,4-Pentanetrione (or IUPAC name pentane-2,3,4-trione, triketopentane or dimethyl triketone) is the simplest linear triketone, a ketone with three C=O groups. It is an organic molecule with formula CH3COCOCOCH3.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Pentane-2,3,4-trione | |
| Other names
Pentanetrione Dimethyltriketone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H6O3 | |
| Molar mass | 114.100 g·mol−1 |
| Appearance | red-orange oil |
| Related compounds | |
Related triketones |
2,3,4-Triketohexane, 2,3,5-Triketohexane, 2,3,5-Triketohexane, 2,4,6-Triketoheptane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Production
2,3,4-Pentanetrione can be made by oxidizing 2,4-pentanedione with selenium dioxide.[2] Ludwig Wolff made the 2,3 oxime by reacting hydroxylamine with isonitrosoacetylacetone in cold, aqueous, concentrated solution.[3]
Yet other ways to make triones start from a β-dione, and oxidise the carbon at the α position, between the two ketone groups. Different methods include reaction with bromine to make a dibromide, and then reacting with acetaldehyde and hydrolysing.[4] Nitrogen oxides can also be used. Another way is by reacting 2,4-pentandedione with p-nitroso-N,N-dimethylaniline; forming an α-diazo-β-dicarbonyl derivative compound, and then reacting that with triphenylphosphine, and then hydrolyzing with sodium nitrite solution. Or the α-diazo-β-dicarbonyl derivative can be treated with tert-butylhypochlorite.[4]
Properties
2,3,4-Pentanetrione appears as an orangy-red oil.[1] The more intense colour is due to the greater conjugation of double bonds, and potential resonance.[5] The type of colour centre is called a dependent chromophore, as when there are fewer C=O groups the colour is less intense or absent. So diacetyl with two carbonyls groups is yellow, and acetone with one has no colour.[6]
At a reduced pressure of 20 mmHg it boils at 60 °C. It is hygroscopic.[1]
The odour is strong and not bad.[7]
Reactions
On absorbing water, perhaps from the atmosphere, 2,3,4-pentanetrione forms a hydrate. The hydrate melts at 52 °C.[1] The hydrate with formula CH3COC(OH)2COCH3 is colourless.[8]
2,3,4-Pentanetrione is a strong reducing agent.[9] Thermal decomposition catalyzed by copper sulfate results in carbon dioxide and diacetyl.[9]
2,3,4-Pentanetrione reacts with hydrogen peroxide to yield acetic acid and carbon monoxide. Adding HOOH between C2 and C4 yields a cyclic intermediate which decomposes.[10]
With light or organic peroxides, a free radical reaction can take place (as with triketones), whereby the molecule is slit into CH3COCO• and CH3CO• fragments. These can combine with intact molecules or each other to form other ketones.[11]
When heating 2,3,4-pentanetrione with oxygen, the oxidation products are diacetyl, acetic acid, water and carbon dioxide.[9]
Alkalis convert it into acetic acid and formaldehyde.[8]
2,3,4-Pentanetrione does a condensation reaction with o-phenylenediamine to yield methyl-quinoxaline-2-methylketone, a heterocyclic dicycle. In this the carbon number 2 and 3 react with the amine groups, leaving the CO on number 5 alone.[12]
Another condensation can happen with 2,5,6-triamino-4(3H)pyrimidinone giving 6-acetyl-2-amino-7-methyl-4(3H)pteridinone.[13]
Grignard agents react. Phenyl magnesium bromide in excess reacting with 2,3,4-pentanetrione gives phenylacetylcarbinol and also methyldiphenylcarbinol.[14]
Derivatives
Derivatives include 2,3,4-pentanetrione-3-oxime, 2,3,4-pentanetrione-3-(O-methyloxime), 2,3,4-pentanetrione-2-oxime, 2,3,4-pentanetrione-2,3-dioxime, 2,3,4-pentanetrionetrioxime, 2,3,4-pentanetrione semicarbazone, 2,3,4-pentanetrione phenylhydrazone, 2,3,4-pentanetrione bis(phenylhydrazone).[1]
The 3-oxime melts at 75 °C and the bis(semicarbazone) has a melting point of 221 °C.[8]
References
- Townshend, A.; Burns, D. T.; Lobinski, Ryszard; Newman, E. J.; Guilbault, G.; Marczenko, Z.; Onishi, H. (1993). Dictionary of Analytical Reagents. CRC Press. p. 766. ISBN 9780412351501.
- Arora, Amit (2006). Carbohydrates And Proteins. Discovery Publishing House. p. 145. ISBN 9788183561785.
- Wolff, Ludwig (1902). "Ueber Diazoanhydride". Justus Liebig's Annalen der Chemie. 325 (2): 194. doi:10.1002/jlac.19023250202.
- Dayer, Francis; Dao, Huu L?; Gold, Hellmut; Rod?-Gowal, Heike; Dahn, Hans (6 November 1974). "Zur Herstellung von 1,2,3-Tricarbonylverbindungen aus 1,3-Dicarbonylverbindungen. 27. Mitteilung ?ber Reduktone und Tricarbonylverbindungen [1]". Helvetica Chimica Acta. 57 (7): 2201–2209. doi:10.1002/hlca.19740570736.
- Panda, H. (2016). Modern Technology of Textile Dyes & Pigments (2nd Revised ed.). Niir Project Consultancy Services. pp. 85–86. ISBN 9789381039717.
- Sharma, Dr B. K. (1981). Spectroscopy. Krishna Prakashan Media. p. S-125. ISBN 9788182830189.
- Calvin, Melvin; Wood, C. L. (November 1940). "Conjugation of Carbonyl Groups and the Absorption Spectrum of Triketopentane". Journal of the American Chemical Society. 62 (11): 3152–3155. doi:10.1021/ja01868a072.
- von Richter, Victor (1934). Organic chemistry; or, Chemistry of the carbon compounds. p. 73. doi:10.1002/jctb.5000534313.
- Arnett, Edward M.; Mendelsohn, Morris A. (October 1962). "Destructive Autoxidation of Metal Chelates. IV. Kinetics and Mechanism". Journal of the American Chemical Society. 84 (20): 3824–3829. doi:10.1021/ja00879a008.
- Borowski, Tomasz; Bassan, Arianna; Siegbahn, Per E.M. (October 2006). "DFT study of the uncatalyzed dioxygenation of acireductone". Journal of Molecular Structure: THEOCHEM. 772 (1–3): 89–92. doi:10.1016/j.theochem.2006.06.025.
- Urry, W. H.; Pai, Mei-Shu H.; Chen, C. Y. (December 1964). "Acyl Exchange Reactions of Vicinal Triones". Journal of the American Chemical Society. 86 (23): 5342–5343. doi:10.1021/ja01077a066.
- Chesseman, G. W. H.; Cookson, R. F. (2009). The Chemistry of Heterocyclic Compounds, Condensed Pyrazines. John Wiley & Sons. p. 130. ISBN 9780470188859.
- Brown, D. J. (2009). The Chemistry of Heterocyclic Compounds, Fused Pyrimidines: Pteridines. John Wiley & Sons. p. 89. ISBN 9780470188378.
- Reuben F. Miller (1952). The Reaction of Organomagnesium Compounds With Triketones (Thesis). Louisiana State University and Agricultural & Mechanical College.