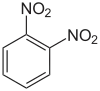
1,2-Dinitrobenzene
1,2-Dinitrobenzene is an organic compound with the formula C6H4(NO2)2. It is one of three isomers of dinitrobenzene. The compound is a white or colorless solid that is soluble in organic solvents. It is prepared from 2-nitroaniline by diazotization and treatment with sodium nitrite in the presence of a copper catalyst.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,2-Dinitrobenzene | |
| Other names
ortho-dinitrobenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.666 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 3443 1597 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H4N2O4 | |
| Molar mass | 168.108 g·mol−1 |
| Appearance | white solid |
| Density | 1.565 g/cm3 |
| Melting point | 118 °C (244 °F; 391 K) |
| Boiling point | 318 °C (604 °F; 591 K) |
| Hazards | |
| GHS labelling: | |
 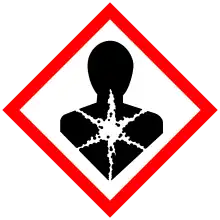 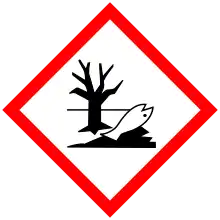 | |
| Danger | |
| H300, H310, H330, H373, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+P310, P302+P350, P304+P340, P310, P314, P320, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- E. B. Starkey (1939). "p-Dinitrobenzene". Org. Synth. 19: 40. doi:10.15227/orgsyn.019.0040.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.