(Terpyridine)ruthenium trichloride
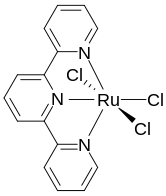
(Terpyridine)ruthenium trichloride is the coordination complex with the formula RuCl3(terpy), where terpy is terpyridine. It is a brown paramagnetic solid that is a precursor to other complexes of ruthenium, mainly by substitution of the chloride ligands. The complex has octahedral geometry.[1] The synthesis of this complex was reported for the first time in 1980, it was prepared by mixing ruthenium trichloride and terpyridine in ethanol, heating the mixture to reflux conditions[2] A later synthetic protocol uses a similar approach by heating ruthenium trichloride with a DMF solution of terpyridine.[3]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C15H11Cl3N3Ru | |
| Molar mass | 440.69 g·mol−1 |
| Appearance | brown solid |
| Density | 1.451 g/cm3 |
| insoluble | |
| Hazards | |
| GHS labelling: | |
| Warning | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Ziegler, Marco; Monney, Véronique; Stoeckli-Evans, Helen; von Zelewsky, Alex; Sasaki, Isabelle; Dupic, Gilles; Daran, Jean-Claude; Balavoine, Gilbert G. A. (1999). "Complexes of New Chiral Terpyridyl Ligands. Synthesis and Characterization of Their Ruthenium(II) and Rhodium(III) Complexes". Journal of the Chemical Society, Dalton Transactions (5): 667–676. doi:10.1039/a900194h.
- Sullivan, B. Patrick; Calvert, Jeffrey M.; Meyer, Thomas J. (May 1980). "Cis-trans isomerism in (trpy)(PPh3)RuC12. Comparisons between the chemical and physical properties of a cis-trans isomeric pair". Inorganic Chemistry. 19 (5): 1404–1407. doi:10.1021/ic50207a066. ISSN 0020-1669.
- Bessel, Carol A.; Leising, Randolph A.; Szczepura, Lisa F.; Perez, Willie J.; Vo Huyhn, My Hang; Takeuchi, Kenneth J. (1998). Trichloro[2,2':6',2-terpyridine]ruthenium(III) and Phosphine Ligand Derivatives. Inorganic Syntheses. Vol. 32. p. 186-198. doi:10.1002/9780470132630.ch32.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.