The States of Matter

There are four main states of matter:
| Solids | Solids hold their shape rigidly. | Examples: Blocks, dishes, chairs, pencils |
|---|---|---|
| Liquids | Liquids take the shape of their container, but hold themselves together. | Examples: Water, soda, milk, blood |
| Gases | Gases take the shape of their container completely, spreading apart. | Examples: Air, oxygen, carbon dioxide, steam |
| Plasmas | Plasmas can act like gases, but are also affected by electromagnetic fields. | Examples: Candle flames, lightning bolts, stars. |
Each state is also known as a phase. Elements can change from one phase to another under certain physical conditions. These conditions are temperature and pressure. Temperature is related to the amount of heat a substance has, and pressure is the amount of force "squeezing" the substance. Deep underwater there is high pressure, and atop a mountain there is low pressure.
There is also another state of matter called plasma. It exists on the sun and in fluorescent lights. Plasma is basically a gas that has become electrified.
There are special names for the process of changing from one phase to another:
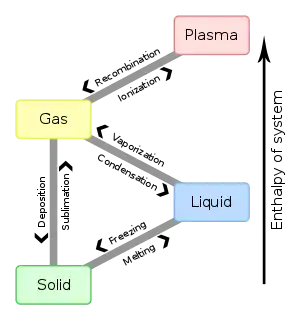
A substance can change from one phase to another, but still be the same substance. You can see water vapor over a boiling pot of water. That gas can condense and become a drop of water. If you put that drop in the freezer, it would become a solid. No matter what phase it is in, it is always the same chemical: water. It has the same chemical properties. In other words, ice is just solid water, and steam is just gaseous water.