Water gas reaction happens at elevated temperatures and pressures between water and carbon (usually coke or coal):
H2O + C -> H2 + CO
The product of this is called synthesis gas and can be extremely useful in bonding with metallic heterogeneous catalysts. For example, in the Fischer-Trospsch process, transition metals are used as catalysts to speed up these reactions like H2 + CO -> Alkanes with Co as the catalyst. 3H2 + CO -> CH4 + H2O with Ni as a catalyst. 2H2 + CO -> CH3OH with Zn/Cu as a catalyst. Ni catalyst is used in a process called steam reforming where methane is mixed with steam at high temperature to create hydrogen gas and carbon monoxide.
Some examples are:
Methane: CH4 + H2O (+heat) → CO + 3H2
Propane: C3H8 + 3H2O (+heat) → 3CO + 7H2
Ethanol: C2H5OH + H2O (+heat) → 2CO + 4H2
Gasoline: C8H18 + 8H2O (+heat) → 8CO + 17H2 C7H8 + 7H2O (+heat) → 7CO + 11H2
In a water gas shift reaction, CO + H2O -> CO2 + H2 with the addition of Zn-Cu as the catalyst. This reaction is thermodynamically favorable at about 400 degrees Celsius.
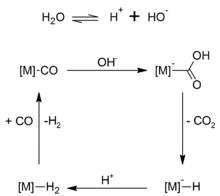
References
http://www1.eere.energy.gov/hydrogenandfuelcells/production/natural_gas.html Miessler, Gary. Inorganic Chemistry. 4th Edition.