Glycolysis is the first step of glucose catabolism. Glycolysis is divided into two categories: aerobic (chemical reactions that occur with the presence of oxygen) and anaerobic (chemical reactions that do not require oxygen). An example of anaerobic glycolysis is fermentation.
Glucose is the reactant; while ATP and NADH are the products of the Glycolysis reaction. There are three stages in an aerobic glycolysis reaction: 1) decarboxylation of pyruvate 2) Citric Acid Cycle (also known as the Krebs Cycle) 3) Electron transport chain.
Glycolysis consists of a total of 10 chemical reactions that starts with the breakdown of glucose into pyruvate and NADH which takes place in the cytoplasm.
- Step 1: Phosphorylation of Glucose
- This reaction is irreversible under intracellular condition, transferase class
- Catalyzed by hexokinase-soluble and cytosolic protein (glucokinase in liver)
- Requires ATP, and Mg2+ as substrates, generates ADP
- ΔG′°= -16.7 kJ/mol
- Step 2: Conversion of Glucose 6-Phosphate to Fructose 6-Phosphate
- This reaction is reversible, isomerase class
- Catalyzed by phosphohexo isomerase
- Requires Mg2+ as a substrate
- ΔG′°= 1.7 kJ/mol
- Step 3: Phosphorylation of Fructose 6-Phospate to Fructose 1,6-Bisphosphate
- Irreversible reaction, transferase class
- Catalyzed by phosphofructokinase-1 which is highly regulate allosteric enzyme
- Requires ATP, and Mg2+ as substrates, generates ADP
- ΔG′°= -14.2 kJ/mol
- Step 4: Cleavage of Fructose 1,6-Bisphosphate
- Reversible reaction, lyase class
- Catalyzed by aldose
- Yields 2 different triose phosphates: G3P (an aldose), and DHAP (a ketose)
- ΔG′°= 23.8 kJ/mol
- Step 5: Interconversion of the Triose Phosphate
- Reversible reaction, isomerase class
- Catalyzed by triose phosphate isomerase
- ΔG′°= 7.5 kJ/mol
- Step 6: Oxidation of Glyceraldehyde 3-Phosphate to 1,3-Bisphosphoglycerate
- Reversible reaction, oxidoreductase class
- Catalyzed by glyceraldehyde 3-phosphate dehydrogenase
- Requires NAD+, yields NADH
- ΔG′°= 6.3 kJ/mol
- Step 7: Phosphoryl Transfer from 1,3-Bisphosphoglycerate to ADP
- Reversible reaction, transferase class
- Catalyzed by phosphoglycerate kinase
- Requires ADP and Mg2+, generates ATP
- ΔG′°= -18.5 kJ/mol
- Step 8: Conversion of 3-Phosphoglycerate to 2-Phosphoglycerate
- Reversible reaction, isomerase class
- Catalyzed by phosphoglycerate mutase
- Requires Mg2+
- ΔG′°= 4.4 kJ/mol
- Step 9: Dehydration of 2-phosphoglycerate to Phosphoenolpyruvate
- Reversible reaction,, lyase class
- Catalyzed by enolase
- ΔG′°= 7.5 kJ/mol
- Step 10: Transfer of the Phosphoryl Group from Phosphoenolpyruvate to ADP
- Irreversible reaction, transferase class
- Catalyzed by pyruvate kinase
- Requires ADP, Mg2+, K+, generates ATP
- ΔG′°= -31.4 kJ/mol
- The product pyruvate first appears in enol form, then tautomerizes to keto form.
The net reaction for Glycolysis is :
Glucose+2ADP+2P1+2NAD+ --> 2 Pyruvate + 2ATP+2NADH+2H++2H2O
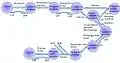 Figure 1 briefly illustrates the steps in glycolysis Reference : en.wikipedia, original author- Tekks.
Figure 1 briefly illustrates the steps in glycolysis Reference : en.wikipedia, original author- Tekks.
Fermentation Glycolysis in fermentation occurs under anaerobic condition, thus, NAD+ has to be regenerated. In order to do obtain NAD+, pyruvate is reduced into ethanol or lactic acid. During fermentation, only 2 ATP per glucose are produced; therefore, it is not too efficient. There are two types of fermentation: 1) Alcohol fermentation which occurs in yeast and some bacteria and 2) Lactic acid fermentation which occurs in some fungi and bacteria, and muscles cells.