Metallic bonds occur among metal atoms. Whereas ionic bonds join metals to non-metals, metallic bonding joins a bulk of metal atoms. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action.
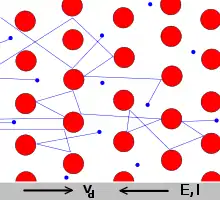
When metallic bonds form, the s and p electrons delocalize. Instead of orbiting their atoms, they form a "sea of electrons" surrounding the positive metal ions. The electrons are free to move throughout the resulting network. The delocalized nature of the electrons explains a number of unique characteristics of metals:
| Metals are good conductors of electricity | The sea of electrons is free to flow, allowing electrical currents. |
| Metals are ductile (able to draw into wires) and malleable (able to be hammered into thin sheets) |
As the metal is deformed, local bonds are broken but quickly reformed in a new position. |
| Metals are gray and shiny | Photons (particles of light) cannot penetrate the metal, so they bounce off the sea of electrons. |
| Gold is yellow and copper is reddish-brown | There is actually an upper limit to the frequency that is reflected. It is too high to be visible in most metals, but not gold and copper. |
| Metals have very high melting and boiling points | Metallic bonding is very strong, so the atoms are reluctant to break apart into a liquid or gas. |
Metallic bonds can occur between different elements. A mixture of two or more metals is called an alloy. Depending on the size of the atoms being mixed, there are two different kinds of alloys that can form:
 Substitutional alloy
Substitutional alloy Interstitial alloy
Interstitial alloy
The resulting mixture will have a combination of the properties of both metals involved.