Silicates are the largest and most important group of minerals in nature. Silicates account for around 95% of all the rocks of the Earth's crust, and the soils, clays and sands formed when those rocks break down.
Silicates are so ubiquitous that oxygen is the most abundant element in the Earth's crust by mass, with silicon in second place: oxygen makes up 46% of the mass of the Earth's crust, while silicon is responsible for 27%. Silicon and oxygen together therefore account for 73% of the crust.
Tetrahedra
The basic building block of all silicate minerals is the [SiO4]4− tetrahedron. There are four covalent Si−O bonds. Each oxygen atom forms one vertex of the tetrahedron. The silicon to oxygen ratio is 1:4.
Silicate minerals containing isolated [SiO4]4− tetrahedra are called nesosilicates or orthosilicates.
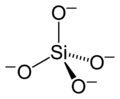
structural formula 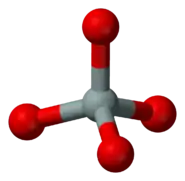
ball-and-stick model 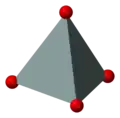
polyhedral model 
Chemical Ideas representation
Corner-sharing tetrahedra
If two [SiO4]4− tetrahedra share an oxygen atom at one common vertex, an [Si2O7]6− ion is formed. The silicon to oxygen ratio is 2:7.
Silicate minerals containing isolated [Si2O7]6− double tetrahedra are called sorosilicates.
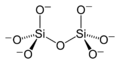
structural formula 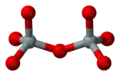
ball-and-stick model 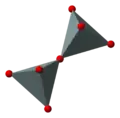
polyhedral model 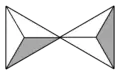
Chemical Ideas representation
Chains
Silicate minerals containing chains are termed inosilicates.
Single chains
(SiO32−)n. The silicon to oxygen ratio is 1:3.
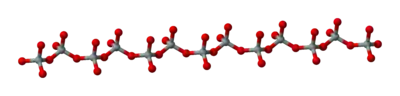
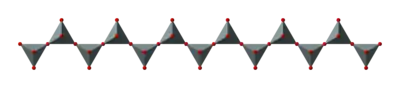

Double chains
(Si4O116−)n. The silicon to oxygen ratio in double chains is 4:11.
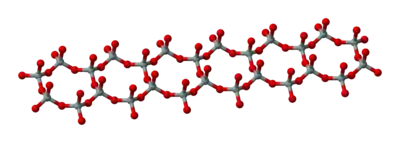
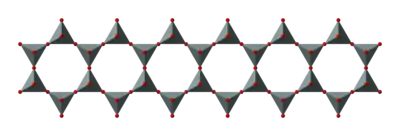
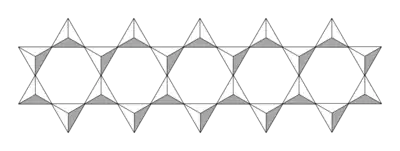
Asbestos
Asbestos (from Greek ἅσβεστος, unquenchable) is a group of fibrous silicate minerals containing double chains. Prolonged exposure to dust containing fibres from certain types of asbestos is now known to cause scarring of the lungs, lung cancer, and a particularly aggressive cancer called mesothelioma. Mesothelioma is almost always fatal, with a median survival time of 11 months. Due to the exceptional danger posed by some absestos, all work involving asbestos in the UK must be done by specialist companies. The vast majority of asbestos is the so-called white form, which is not known to pose any real danger.
Sheets
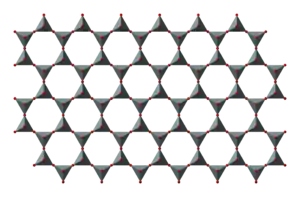
SiO4 tetrahedra can be arranged to form sheets, as illustrated to the right. The formula of such a sheet can be written (Si2O52−)n.
Silicate minerals containing sheets are termed phyllosilicates.
Three-dimensional frameworks
Perhaps the most structurally complicated silicates are those based on networks of Si and O that extend in all three dimensions. Examples of such minerals include quartz, zeolites and feldspars.
Silicate minerals containing three-dimensional frameworks are termed tectosilicates.