< A-level Chemistry < OCR (Salters)
Nitrile hydrolysis proceeds by the mechanism outlined below. It begins with protonation of the nitrile nitrogen, which increases the partial positive charge on carbon and therefore renders carbon more susceptible to nucleophilic attack by a water molecule. Addition of water to the nitrile is the key step in nitrile hydrolysis − the next few steps are simple proton transfers, until an amide is formed. The amide then typically undegoes further hydrolysis to a carboxylic acid.
Summary
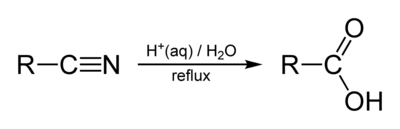
Step 1: Protonation

Step 2: Nucleophilic attack of water at carbon
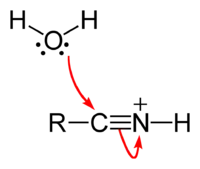
Step 3: Intramolecular proton transfer
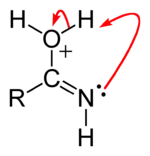
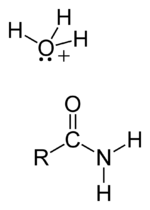
Step 4: Deprotonation

Step 5: Amide formed
This article is issued from Wikibooks. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.